Significance
Carnivorous pitcher plants have thus far been considered a classic example of passive, motionless pitfall traps. Here, we describe a rapid, passive-dynamic movement used by an Asian Nepenthes pitcher plant to capture insect prey. We show that the pitcher lid functions as a rain-driven torsion spring that combines material properties adapted for momentum transfer with a unique friction-reducing surface coating. Unlike other pitcher traps, this capture mechanism employs rapid movement. It also differs from the metabolically costly mechanisms of active carnivorous plants such as Venus flytraps because the movement is externally driven. It therefore establishes a new category of rapid plant movements that challenges the conventional distinction between active and passive carnivorous plant traps.
Keywords: plant movement, biomechanics, carnivorous plants, torsion spring, wax crystals
Abstract
Plants use rapid movements to disperse seed, spores, or pollen and catch animal prey. Most rapid-release mechanisms only work once and, if repeatable, regaining the prerelease state is a slow and costly process. We present an encompassing mechanism for a rapid, repeatable, passive-dynamic motion used by a carnivorous pitcher plant to catch prey. Nepenthes gracilis uses the impact of rain drops to catapult insects from the underside of the canopy-like pitcher lid into the fluid-filled trap below. High-speed video and laser vibrometry revealed that the lid acts as a torsional spring system, driven by rain drops. During the initial downstroke, the tip of the lid reached peak velocities similar to fast animal motions and an order of magnitude faster than the snap traps of Venus flytraps and catapulting tentacles of the sundew Drosera glanduligera. In contrast to these active movements, the N. gracilis lid oscillation requires neither mechanical preloading nor metabolic energy, and its repeatability is only limited by the intensity and duration of rainfall. The underside of the lid is coated with friction-reducing wax crystals, making insects more vulnerable to perturbations. We show that the trapping success of N. gracilis relies on the combination of material stiffness adapted for momentum transfer and the antiadhesive properties of the wax crystal surface. The impact-driven oscillation of the N. gracilis lid represents a new kind of rapid plant movement with adaptive function. Our findings establish the existence of a continuum between active and passive trapping mechanisms in carnivorous plants.
The rapid trap closure of the Venus flytrap prompted Charles Darwin to call it the “most wonderful plant in the world” (1). Arguably the most studied example, it is only one of many cases of plants using rapid movement to trap insects. Often overlooked, the ability to move is as integral to plant life as it is to animals. From the reorientation of leaves and growth axes in response to external stimuli such as light, wind, and gravity (2) to the opening and closing of stomata (3), many plant movements are driven by changes of osmotic pressure. As a result, these movements are limited by the speed of diffusion (4) and often too slow to be observed without the help of time-lapse recordings. In contrast, the rapid movements used to disperse seed (5), spores (6), or pollen (7) as well as the movement-based trapping mechanisms of carnivorous Venus flytraps (8) and bladderworts (9) rely on the rapid release of stored elastic energy. Although these movements can achieve extraordinary speeds (10), they often rely on single-shot mechanisms or, where repeatable, require considerable time and energy to regain the prerelease state.
Carnivorous plant traps are conventionally divided into “active” (moving) and “passive” (immobile) traps. Examples of active traps are the snap traps of Dionaea muscipula (Venus flytrap) and Aldrovanda vesiculosa, the suction traps of bladderworts (genus Utricularia), and the moving flypaper tentacles of Drosera sundews (11). A common feature of these traps is their reliance on rapid movements that are triggered by the prey and activated through electrophysiological signaling processes (12–17). In contrast, passive traps do not move and rely entirely on physical obstructions, slippery surfaces, and sticky secretions to capture prey (11). The pitchers of Asian Nepenthes plants (Fig. 1) are classic examples of passive carnivorous traps.
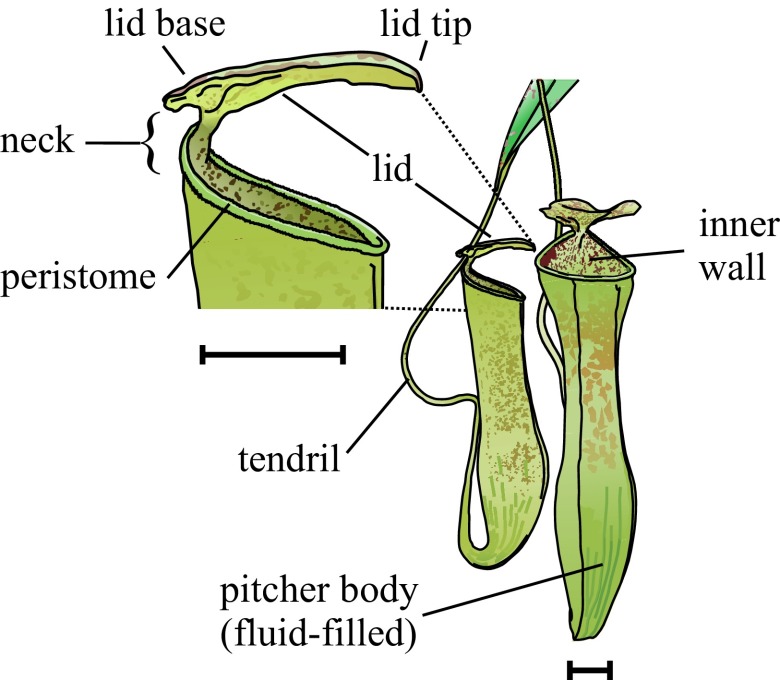
Fig. 1.
Pitcher traps of Nepenthes gracilis. (Scale bars: 1 cm.)
Around 120 Nepenthes species are distributed across the perhumid tropical regions of Southeast Asia (18). Like other carnivorous plants, they are found in habitats where nutrients are scarce but light and water are abundant. The ability to derive nitrogen and phosphorus from trapped insects enables pitcher plants to thrive where most other plants fail. Prey is attracted to pitchers by visual and olfactory cues (19–22) and lured to the slippery trapping surfaces by the secretion of sugary nectar (23–25). The ubiquitous trapping surface is the upper pitcher rim, called the peristome. It offers safe access to nectar when dry but turns into a deadly slide for insects when wet (26, 27). Once trapped, prey is prevented from escaping by the often viscoelastic pitcher fluid (28, 29) or by antiadhesive wax crystal surfaces on the inner pitcher wall (30–32). Fluid viscoelasticity and wax crystal surfaces are only present in a subset of Nepenthes species and are often mutually exclusive (33, 34).
The pitcher lid is generally regarded as a protective structure against flooding by rain. In Nepenthes gracilis, the lid has an additional adaptive function as a trapping device. In this species only, the lower lid surface is covered with a crystalline epicuticular wax layer that superficially resembles the inner wall coating (35). However, the lid wax crystals are structurally different from those on the inner wall. Bauer et al. (35) showed that N. gracilis allocates a higher proportion of its nectar production to the lower lid surface than the sympatric Nepenthes rafflesiana, a conventional peristome-trapping species. Insects can walk upside down under the N. gracilis lid and harvest nectar but they regularly get knocked off by the impact of rain drops and end up as prey in the pitcher. The experimental application of an antislip coating to the lower lid surface significantly reduced the prey capture success of N. gracilis pitchers in the field (35). The same treatment applied to the wax crystal-free N. rafflesiana lids had no effect on prey capture (29), suggesting that the wax crystals are crucial for the trap function of the N. gracilis lid.
The present study unveils an additional and unique adaptation of the N. gracilis lid that goes beyond antiadhesive wax crystals. Starting from the observation that the N. gracilis lid is more rigid than that of N. rafflesiana, we hypothesized that the material properties of the N. gracilis lid are adapted to facilitate prey capture by making use of the mechanical impacts of rain drops. To test this hypothesis, we used high-speed video analysis, noncontact vibration monitoring, and kinematic modeling techniques to investigate whether the impact response of the N. gracilis lid is different from that of the peristome-trapping N. rafflesiana. We further propose that this impact response allows N. gracilis to use momentum from falling rain drops to “flick” insects into the pitcher—making it the only pitcher plant with a dynamic trapping mechanism. Capture trials and friction force measurements with live ants were conducted to disentangle the effects of surface structure and material properties on trap performance.
Results
The Pitcher Lid of N. gracilis Resembles a Stiff Plate Articulated with a Basal Torsional Spring.
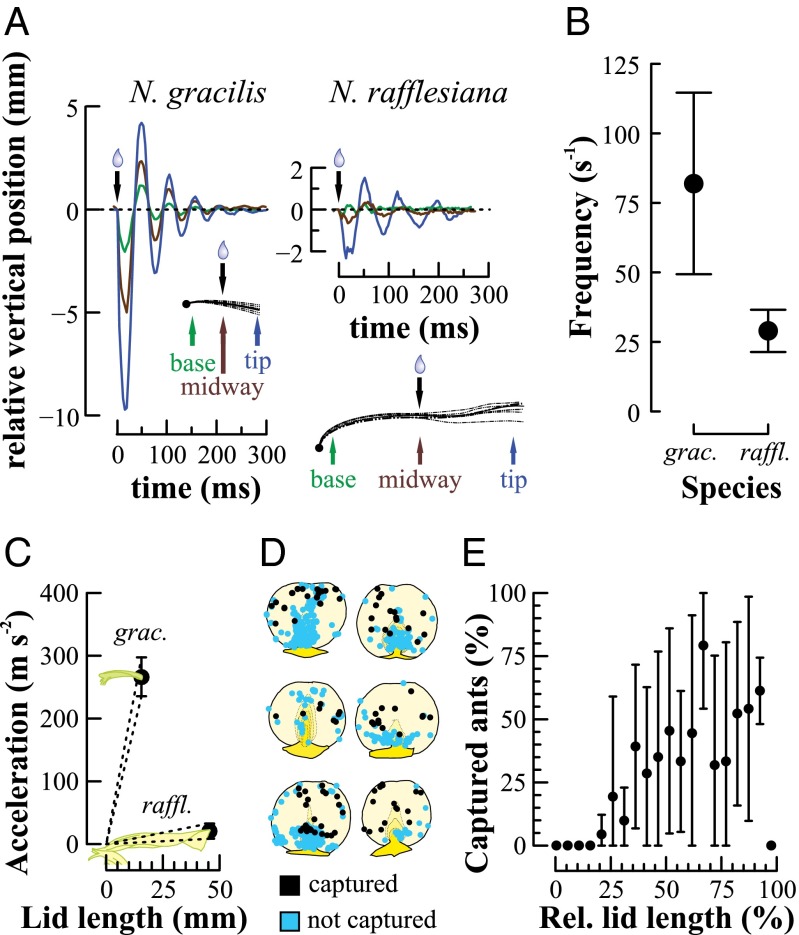
High-speed video recordings revealed that N. gracilis lids (n = 12) responded to drop impacts with harmonic oscillations. The initial amplitude of these oscillations at the lid tip was 3.83 ± 3.09 mm peak to peak (mean ± SD used throughout). The oscillations were highly stereotyped even if the impact location was varied randomly. Three points along each lid (proximal, medial, and distal) moved in phase, establishing that the lid did not bend but pivoted around a flexible hinge point in the pitcher neck (Fig. 2A). In contrast, the larger and more compliant N. rafflesiana lids (n = 10) responded to the drop impact by bending, resulting in hardly any motion of the proximal half and more pronounced movement distally (initial tip amplitude 2.23 ± 0.43 mm). Although the N. rafflesiana lids bent easily, their average rotational stiffness was 2.5 times higher than that of the N. gracilis lids (Table 1). However, the N. gracilis lids are not only smaller (Fig. 2 A and C) but also much lighter, resulting in a significantly faster oscillation in response to similar impacts (81.92 ± 32.73 Hz vs. 21.16 ± 3.06 Hz; Welch’s t test, t = 6.40, df = 11, P < 0.001; Fig. 2B).
Fig. 2.
Mechanical drop impact response of pitcher lids and effect on insect visitors. (A) Impact-driven oscillation of an N. gracilis and an N. rafflesiana lid recorded in the field with a high-speed video camera. (B) Oscillation frequency of N. gracilis (n = 12) and N. rafflesiana (n = 10) lids in response to a rain drop impact. (C) Peak acceleration as a function of position along the length of the lid for both species, estimated from modeling both lids as stiff rectangular prisms rotating about a proximal fulcrum (cf. Fig. S1). (D) Fate of visiting ants following a drop impact, depending on the position under six individual N. gracilis lids. Each circle represents an ant (n = 44–361 ants per lid, 993 ants in total). (E) Capture rate as a function of relative distance from the lid base (in 5% increments) as determined from data in D. In B, C, and E, circles represent means and error bars represent SD. For statistics see text.
Table 1.
Comparison of lid dimensions (length and mass, from direct measurements) and oscillation parameters in response to a simulated rain drop impact (from high-speed video analysis and kinematic modeling) for N. gracilis and N. rafflesiana
| Parameter | N. gracilis (n = 12) | N. rafflesiana (n = 10) | Test | Test statistics | P |
| Lid length, mm | 15.63 ± 2.75 | 44.46 ± 5.49 | Welch | t = 15.11 | <0.001 |
| Lid mass, mg | 82.42 ± 34.67 | 620.60 ± 204.18 | Wilcoxon–Mann–Whitney | U = 0.00 | <0.001 |
| Frequency, Hz | 81.92 ± 32.73 | 21.16 ± 3.06 | Welch | t = 6.40 | <0.001 |
| Maximum acceleration, m⋅s−2 | 267.65 ± 27.60 | 27.60 ± 24.23 | Wilcoxon–Mann–Whitney | U = 1.00 | <0.001 |
| Rotational stiffness, kg⋅m2⋅s−2 | 0.007 ± 0.007 | 0.017 ± 0.009 | Wilcoxon–Mann–Whitney | U = 21.00 | 0.009 |
| Damping, kg⋅m2⋅s−1 | 2.05 ± 1.37 × 10−6 | 3.05 ± 0.72 × 10−5 | Wilcoxon–Mann–Whitney | U = 0.00 | <0.001 |
Trapping Risk Is a Function of Ant Position Under the Lid.
The stiff plate pivoting behavior implies that acceleration and inertial forces acting on insects on the lower lid surface should increase linearly with distance from the pivot point. The peak accelerations calculated for the N. gracilis lids were an order of magnitude higher than those of the N. rafflesiana lids (Fig. 2C). Capture trials with ants on six different N. gracilis lids showed that the likelihood to be dislodged by a drop impact was dependent on the position of the ant under the lid (Fig. 2D). Although there is some morphological variation between the individual lids, ants never fell from the proximal 20% of the lid. The fall frequency increased significantly from the proximal to the distal end of the lid (Page test for trend, n = 6, L = 14475.0, P < 0.001; Fig. 2E). Ants at the very edge of the lid, however, were able to hold on to the edge, making them resistant to falling. We repeatedly observed ants successfully holding on to the edge of a vibrating lid with just one foot whereas others, positioned away from the edge, were dislodged by the same impact. On all observed lids, ants showed a strong preference to position themselves under the thicker, wedge-shaped proximal end of the lid centerline. Ants were rarely dislodged from this position (Fig. 2D).
Rain Drops Exclusively Activate the Pivoting Mode of the N. gracilis Lid.
High-resolution vibration measurements resolved the impact response of an N. gracilis lid in more detail (Fig. 3A). A series of scan points along the length of the lid (Fig. 3B) was shown to oscillate in phase, with the amplitude increasing with distance from the lid base (Fig. 3C), confirming the results from the video analysis. Drop impacts caused a rapid initial downstroke with a sharp change of direction at the lowest point (Fig. 3 C and D). After the initial transient response (Fig. 3D, Top), the lid settled into a regular damped oscillation with a gradual slight decrease of frequency over time due to air resistance (Fig. 3D, Bottom).
Fig. 3.
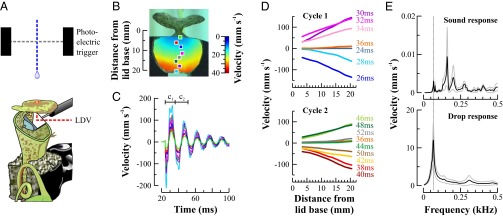
High-resolution vibration analysis of an N. gracilis lid. (A) Experimental setup for measuring the drop impact response of the pitcher lid with a scanning laser Doppler vibrometer (LDV). A front coated mirror was used to deflect the laser beam onto the lower lid surface. (B) Velocity heat map of an impact response showing increasing velocity from lid base to tip. The colored squares highlight eight sample scan points along the centerline of the lid. (C) Time-resolved lid oscillation constructed from sample points in B (c1 and c2, cycles 1 and 2). (D) Deflection shapes of the N. gracilis lid during the first two oscillation cycles following drop impact, showing a linear increase of velocity along the length of the lid. The first cycle is characterized by shorter period and higher velocities than subsequent cycles (cf. C). (E) Frequency spectrum of the lid vibration in response to a 0.1- to 2-kHz sound sweep (Top) and a simulated rain drop impact (Bottom). The black lines represent the average response spectrum calculated for all measurement points, and the gray spectra represent the SD.
Sound-driven oscillations exhibited a resonant frequency of 69 Hz where deflection shapes corroborate the previously described pivoting motion (Fig. 3E, Top, and Movie S1). Additional resonant modes were identified at higher frequencies. Prominent deflection shapes included side-to-side rolling at 148 Hz, level up-and-down movement at 278 Hz, and additional higher-order bending modes at 514 and 925 Hz (Movies S2–S5). Actuating the same lid with simulated rain drops mainly activated the first mode of resonance, pivoting at 69 Hz (Fig. 3E, Bottom, and Movie S6).
A Combination of High Accelerations and Friction-Reducing Wax Crystals Enable N. gracilis to Trap Prey with the Lid.
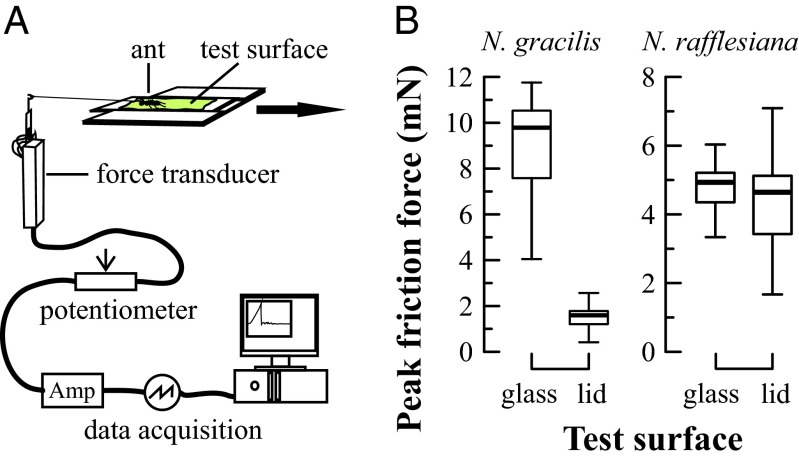
Simulated rain drops dislodged 14 out of 37 ants (38%) from the underside of an N. gracilis lid. In contrast, not a single ant (n = 20) fell from the lid of an N. rafflesiana pitcher or from an N. gracilis lid attached to the underside of an N. rafflesiana lid (n = 39 ants, counting only ants on the N. gracilis part of the lid). This confirms that the rapid vibration of the N. gracilis lid is crucial for its trapping function. Removal of the wax crystals from the lower lid surface of the N. gracilis pitcher reduced the trapping rate drastically to 4% (2 out of 46 ants; Fisher’s exact test, P = 0.002), revealing that the wax crystals are also essential for the trapping function of the N. gracilis lid. Ants consistently generated much lower friction forces on the wax crystal surfaces of three different N. gracilis lids (1.56 ± 0.61 mN) than on a clean glass surface (9.02 ± 2.29 mN; Wilcoxon signed-rank test, n = 11, Z = 2.93, P = 0.003; Fig. 4). In contrast, friction forces on the wax crystal-free undersides of two N. rafflesiana lids (4.38 ± 1.39 mN) were similar to those in control trials on glass (4.73 ± 1.43 mN; Wilcoxon signed-rank test, n = 10, Z = 0.56, P = 0.58; Fig. 4B). The friction forces on glass provided a control to allow comparisons between the measurements on N. gracilis and N. rafflesiana, which were conducted on separate days. This is necessary because absolute friction forces vary depending on shear rate, temperature, and the motivation of the individual (36). From the body mass of the ants (2.15 ± 1.0 mg, n = 48) and the lid acceleration (Fig. 2C) we calculated that the peak inertial force acting on a Crematogaster ant was 0.57 ± 0.27 mN at the tip of an oscillating N. gracilis lid, and 0.03 ± 0.01 mN at the tip of the N. rafflesiana lid.
Fig. 4.
Ant friction forces on pitcher lid surfaces. (A) Experimental setup. (B) Peak friction forces generated by Crematogaster ants on the lower lid surfaces of N. gracilis (n = 3) and N. rafflesiana (n = 2) pitchers in comparison with control surfaces (glass). Bars denote medians, boxes represent the inner two quartiles, and whiskers contain all values within 1.5 times interquartile range. For statistics see text.
Discussion
We show that N. gracilis uses a rapid, rain-powered oscillation of the pitcher lid to capture prey. Although morphologically similar at first sight, the kinematic impact response of the N. gracilis lid (Movie S7) was found to be fundamentally different from that of the N. rafflesiana lid (Movie S8). Adaptations of lid geometry and material properties combined with a friction-reducing wax crystal surface render the N. gracilis lid a highly effective insect trapping device. To our knowledge, this is the only case of an externally driven functional plant movement reported to date.
The torsional spring behavior of the stiff N. gracilis lid results in a pivoting motion. This type of motion entails a linear increase of acceleration along the length of the lid. In contrast, the bending response of the N. rafflesiana lid (Movie S8) results in a whipping motion and leads to a concentration of the acceleration at the very tip of the lid. This does not only limit the surface area where high accelerations are experienced, but also places it at the edge of the lid where insects can get a much better grip by hooking around with their claws. Conversely, the combination of a high bending stiffness along the lid associated with a lower mass and damping coefficient allows the N. gracilis lid to reach higher accelerations spread over a larger surface area. The resulting inertial forces acting on the prey were, in case of the Crematogaster ants, approximately one-third of the measured peak friction forces on the wax crystal-covered lower lid surface. Adhesion forces of smooth insect pads are typically about seven times lower than friction forces on the same surface (37). We would therefore predict the trapping threshold for Crematogaster to be located about halfway along the length of the N. gracilis lid, and closer to the base for ants that have fewer than six feet in contact at the moment of impact. Our observations of trapping locations (Fig. 2 D and E) confirm this prediction. The absence of trapping events from the N. rafflesiana lid (with or without attached wax crystal surface) and from the wax crystal-free N. gracilis lid is also predicted by both measured frictional and calculated inertial forces.
The impact-driven oscillations of the N. gracilis lid are remarkably fast. During the initial downstroke, the tip of the lid reached peak velocities of nearly 1.5 ms−1 and maximum accelerations of almost 300 ms−2, similar to the take-off speed and acceleration of some jumping insects (38). Peak lid velocity is also an order of magnitude faster than the snap traps of the Venus flytrap (8) and the catapulting tentacles of the sundew Drosera glanduligera (15). The only known carnivorous plant movement that exceeds the N. gracilis movement in speed is the rapid opening of the Utricularia suction traps (9, 17). In contrast to these active mechanisms, the N. gracilis lid movement requires neither mechanical preloading nor physiological activation. The fastest reported plant movements—up to 170 ms−1 in extreme cases—are catapult-based mechanisms for seed dispersal (5, 39, 40), spore release (6), and pollen transfer (7, 41). These superfast motions invariably rely on irreversible, single-shot mechanisms. In contrast, the rapid lid movement of N. gracilis can be repeated instantly and indefinitely as long as the external driver, rain drop fall, persists. The finding that rain drops only elicit the trapping-relevant pivoting mode of resonance is testament to the remarkable fine-tuning of the lid properties to this unique mechanism. By filtering out other movement responses, the N. gracilis lid maximizes the downward impact transmission.
Rain plays a key role as an activator for lid and peristome trapping alike (26, 35). In both cases, this leads to a stark bimodality where traps are either switched on or off. The frequent change between dry, safe and wet, dangerous times promotes the survival of worker ants scouting for new food sources, thereby boosting recruitment and ultimately capture rates (42). The erratic nature of rain in the tropical habitats of pitcher plants makes it highly unpredictable. This makes it difficult for insects to avoid rain altogether. Insect activity tends to temporarily decrease during heavy rain (43–46); however, this might not affect capture rates if pitcher visitors are already present at the onset of heavy rain. In addition, N. gracilis might benefit from the tendency of insects to seek shelter from rain on the underside of leaves. Even after the rain has stopped, accumulated water keeps dripping from the vegetation. These usually large drops might lead to further captures once the ants resume their foraging activity.
The lid trapping mechanism of N. gracilis is a striking example of how relatively small evolutionary changes to surface and material properties can add an adaptive function, trapping, to a structure that already serves a completely different purpose, protection from the elements. Hypothetically, similar mechanical adaptations in leaves could enable other plants to literally shake off herbivorous insects during rain. Slippery epicuticular wax crystals are found throughout the plant kingdom and their insect-repellent function has been extensively studied (47). Counterintuitively, highly effective insect-repellent plant surfaces do not necessarily convey increased herbivore resistance because they also exclude most predators and parasitoids, creating a predation-free niche for specialist herbivores that can overcome the antiadhesive barrier (48, 49). Impact- or wetness-dependent insect-repellent mechanisms could provide a strategy to circumvent this tradeoff. More research is needed to investigate the surface structures and impact responses of leaves to potentially exploit passive-dynamic movements for the protection of crop plants.
The use of a rapidly moving plant part for prey capture makes N. gracilis unique among pitcher plants and challenges the conventional divide between active and passive carnivorous traps. Passive prey capture is generally considered motionless, whereas active trapping implies the involvement of metabolically costly, physiological activation mechanisms (11). The externally driven, passive-dynamic movement of the N. gracilis lid represents a novel kind of functional plant movement that is facilitated by specific structural adaptations of the plant but activated and powered exclusively by abiotic factors. The passive-dynamic N. gracilis trap indicates the existence of a continuum between active and passive trapping mechanisms in carnivorous plants.
Materials and Methods
High-Speed Video Analysis.
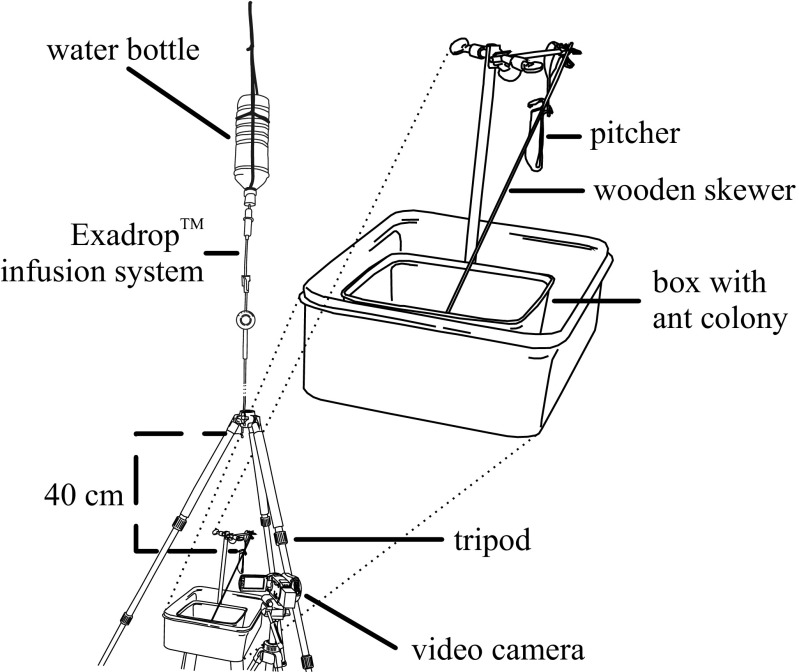
We used high-speed video recordings to investigate the oscillation of N. gracilis (n = 12) and N. rafflesiana (n = 10) lids. Each pitcher was collected from a field site in Brunei Darussalam, transferred to a nearby field station, and mounted on a laboratory stand to resemble the natural orientation on the plant. The impact of rain was simulated with an infusion drip system (Exadrop; B. Braun) releasing droplets from a height of 40 cm centrally above the pitcher lid, with a drip frequency of 0.25–0.35 Hz. The experiments were conducted on an open porch and the setup was subject to natural airflow, causing the location of the drop impact on the lid to vary randomly. A Basler A602f high-speed video camera (Basler AG) with a 60-mm macro lens (Nikon) was mounted at the same height as the lid, providing a 90° side view of the oscillation. A ruler was positioned in the frame to provide a scale. We recorded three oscillations per pitcher at frame rates ranging from 250 to 457 frames per second.
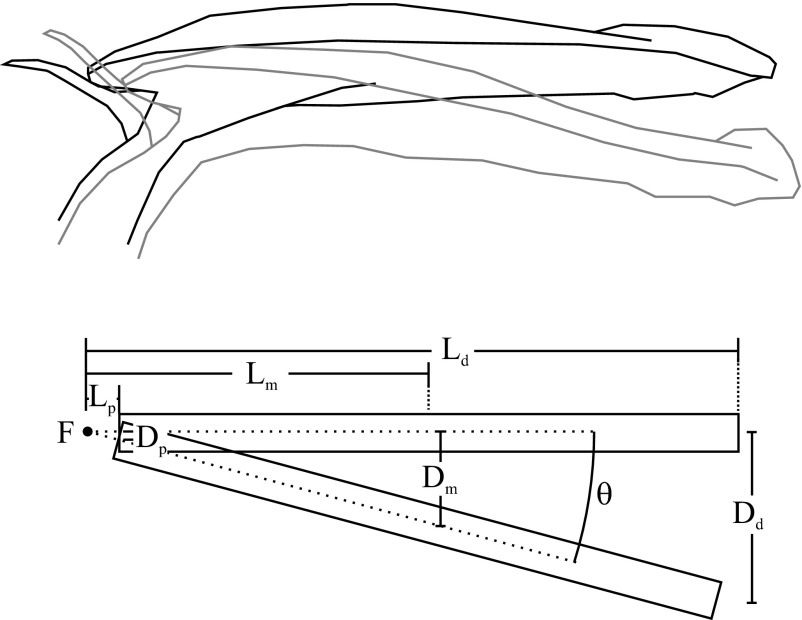
In each video, three points on the lid (proximal, medial, and distal) were tracked manually using a custom-built MATLAB code (The MathWorks, Inc.) (Dataset S1). Frequency was calculated directly from the number of oscillations per time, averaging across replicate recordings and tracking positions. Kinematic modeling techniques in the software Mathematica 5.0 (Wolfram Research) were used to calculate rotational stiffness, damping, velocity, and acceleration from the point tracking results, approximating the lid movement as a rotation of a stiff rectangular beam about a fulcrum located at one end (Fig. S1). For each tracking position i and pitcher p, we calculated the angle of deflection θi,p defined as
| [1] |
Fig. S1.
Illustration of the kinematic model (Bottom) developed to represent the N. gracilis lid (Top). The lid was modeled as a rectangular prism rotating about a fulcrum (F). The angle of displacement (θ) was measured by averaging the angles of displacement of the proximal, medial, and distal point on each leaf. Each angle was calculated by taking the inverse tangent of the displacements (Dp, Dm, and Dd for the proximal, medial, and distal point, respectively) divided by the distance of that point from the fulcrum (Lp, Lm, and Ld). Individual angle measurements had small errors that were smoothed out by averaging the three measurements.
where d is the vertical displacement from neutral and L is the distance from the center of rotation to the tracking point. To minimize the effect of measurement errors, we averaged across recordings before the calculation of θi,p, and then across tracking positions to obtain an angle of deflection θp as a function of time for each individual pitcher. The resulting smoothed curve of angular deflection over time was fit to the equation
| [2] |
where I is the moment of inertia, µ is the damping coefficient, and k is the stiffness of the lid. I was calculated from the mass and length of the lid (mass × length2/3) and µ and k were fit to match the resonant frequency and damping rate of the oscillation. Each model fit returned a function of angle over time. The maximum angular velocity () and acceleration () were derived by differentiating and locating the maxima of the resulting curves. Multiplication by lid length yielded the corresponding linear velocity and acceleration values. The Mathematica code used for the kinematic modeling can be requested from the authors.
Laser Vibrometry.
An N. gracilis plant was obtained from Kew Gardens in London and kept in a climate-controlled room between 30 °C/60% relative humidity (day) and 24 °C/80% relative humidity (night). A scanning laser Doppler vibrometer (PSV-400; Polytec GmbH) was used to measure lid oscillations of a live pitcher in the laboratory. The complete setup including the plant was placed on a vibration-free table (model 784–443-12R; Technical Manufacturing Corp.). Resonant frequencies and mode shapes were identified by recording the response of the lid to repeated 0.1- to 2-kHz sound sweeps with a data acquisition rate of 5.12 × 104 Hz. The pitcher (fluid removed) was mounted so that the lid was orientated vertically, tip pointing down, at 90° to the optical axis of the laser source. Sound from a loudspeaker (bass, 30-cm diameter, positioned outside the vibration isolation table) was used to acoustically drive lid motion. Lid vibrations were monitored across a grid of 98 measurement points. Mechanical responses were recorded in both frequency and time domains using Polytec PSV v.8.2 software.
We recorded the simulated rain drop impact response of the same pitcher using a similar setup as described above for the video analysis. Because the pitcher was now mounted upright, we used a front-coated mirror to deflect the laser beam onto the lid surface from underneath (Fig. 3A). Owing to spatial constraints it was not possible to position the mirror so that the proximal 20% of the lid could be scanned. The remaining lid area was scanned across a grid of 62 measurement points. The focusing distance for each scan point was determined before the scan. During the scan, individual measurements were triggered using a photoelectric sensor positioned in the drop fall path. The sensor was orientated so that the trigger beam was perpendicular to the midrib and crossed the midway point along the length of the lid.
Ant Capture Trials.
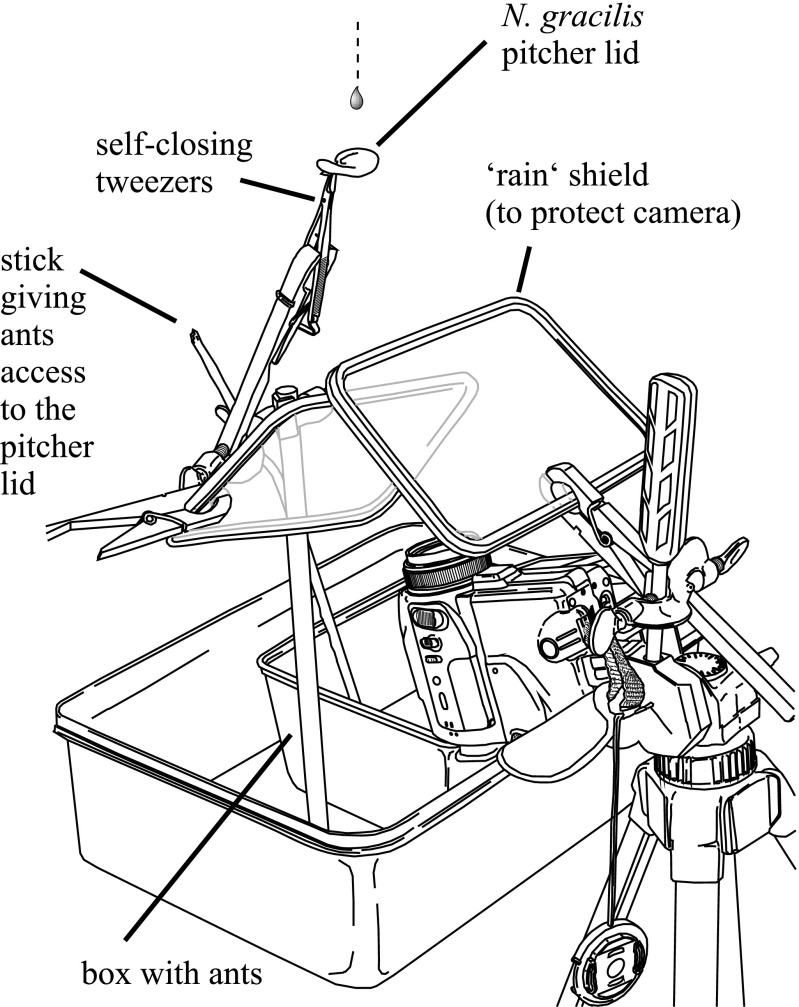
Running experiments with Crematogaster sp. ants on lids of N. gracilis and N. rafflesiana were performed in a field station in Brunei Darussalam. Carton nests with partial ant colonies were collected from the same field site as the pitchers and were kept in open plastic containers with a slippery polytetrafluoroethylene wall coating (Fluon; AGC Chemicals). The ants were fed with honey water and the nests were sprayed regularly with rain water. A freshly collected pitcher was mounted in a natural, upright orientation and the ants were given access via a wooden skewer. We waited 5–10 min to allow the ants to start foraging before simulating rain with a drip system as described above. A Sony DCR-PC120E video camera was used to film the underside of the lid (Fig. S2). Videos were analyzed by counting the number of dislodged ants relative to the number of visitors, defined as an ant fully entering the underside of the lid with all six feet. We tested the performance of ants on the undersides of (i) an N. gracilis lid, (ii) the same lid after removing the wax crystal layer by gently wiping the surface with a soft cloth until it appeared glossy, (iii) an N. rafflesiana lid, and (iv) an unmanipulated N. gracilis lid that had been cut at the base and glued centrally under the lid of an N. rafflesiana pitcher. In the latter case, only ants that fully entered the wax crystal-bearing N. gracilis surface were counted as visitors.
Fig. S2.
Experimental setup to test the impact resistance of foraging Crematogaster ants on the underside of pitcher lids of N. gracilis (with and without wax crystals) and N. rafflesiana (with and without attached wax crystal surface). The ants were allowed to forage freely on the pitcher before we started simulating rain by releasing droplets from a drip infusion system suspended above the pitcher. Observations were recorded with a conventional video camera. Modified from ref. 35.
A second series of capture trials was performed to investigate whether the probability of an ant to be dislodged depended on its location under the N. gracilis lid. To be able to film almost directly from underneath, we cut a lid complete with the pitcher neck and a small section of the rear pitcher wall and mounted it in a horizontal orientation (Fig. S3). Two plastic container lids mounted at an angle, with a narrow gap in between to film through, formed a splash protection for the video camera. Ants were given access to forage on the lid nectar and rain was simulated as previously described. The videos were analyzed by comparing the last still image before and the first one after each drop impact and mapping the thorax positions of dislodged and “successful” ants on the underside of the lid. The experiment was repeated with six individual N. gracilis lids.
Fig. S3.
Experimental setup to determine the trapping rate depending on the location of visiting ants under the N. gracilis pitcher lid. Rainfall was simulated with a drip infusion system suspended above the pitcher lid. Ants were allowed to forage for several minutes before the drip was started.
Friction Force Measurements.
Friction forces of Crematogaster sp. ants on the lower lid surfaces of three N. gracilis lids (n = 11 ants) and two N. rafflesiana lids (n = 10 ants) were measured against a control (a smooth, clean, glass surface) using a one-dimensional bending beam force transducer with two semiconductor strain gauges (SS-060-033-500PU-S1; Micron Instruments) in half-bridge configuration and a wire hook on the free end (Fig. 4A). Each ant was tethered to the hook on the bending beam using a human hair glued to the thorax and placed on the test surface, which was then slowly and steadily pulled away from the beam. Only trials where the ants remained stationary and used all six legs to hold on to the surface were considered valid. To account for behavioral variation, we performed six valid measurements for each ant and used means for the data analysis.
Pitchers were collected in the field immediately before the measurements. Test surfaces were prepared by cleaning new glass microscope slides with ethanol and allowing them to air-dry. They were then either used directly as control surfaces or a freshly abscised lid (or lid section, in the case of N. rafflesiana) was superglued flat onto the slide, underside facing up. Care was taken not to damage the wax crystals when mounting N. gracilis lids. In between trials, pitcher surfaces were kept on moist tissue paper in a closed Petri dish. The plant surfaces were replaced as soon as the edges started to roll upwards, indicating the onset of drying.
Supplementary Material
Acknowledgments
We thank Ulmar Grafe for help with obtaining field work permits and Universiti Brunei Darussalam and Brunei Forestry Department for granting these. The families of Hasnan Bin Engin and Hj. Abdul Hadzid Tinggal provided outstanding hospitality during our time in Brunei. We thank the Royal Botanical Gardens at Kew for loaning us the N. gracilis plant used for the laser vibrometry. Robert Malkin, Dominic Clarke, and Thorin Jonsson helped with experiments in the laboratory. The work also benefited from fruitful discussions with Walter Federle. We kindly acknowledge funding from The Leverhulme Trust and the Cambridge Philosophical Society (U.B.), the Biotechnology and Biological Sciences Research Council (D.R.), and The Royal Society (G.P.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1510060112/-/DCSupplemental.
References
- 1.Darwin C. Insectivorous Plants. Appleton, London: 1875. [Google Scholar]
- 2.Gilroy S, Masson P. Plant Tropisms. Blackwell; Ames, IA: 2008. [Google Scholar]
- 3.Zeiger E, Farquhar GD, Cowan IR. Stomatal Function. Stanford Univ Press; Stanford: 1987. [Google Scholar]
- 4.Forterre Y. Slow, fast and furious: Understanding the physics of plant movements. J Exp Bot. 2013;64(15):4745–4760. doi: 10.1093/jxb/ert230. [DOI] [PubMed] [Google Scholar]
- 5.Swaine M, Beer T. Explosive seed dispersal in Hura crepitans L.(Euphorbiaceae) New Phytol. 1977;78(3):695–708. [Google Scholar]
- 6.Noblin X, et al. The fern sporangium: A unique catapult. Science. 2012;335(6074):1322. doi: 10.1126/science.1215985. [DOI] [PubMed] [Google Scholar]
- 7.Edwards J, Whitaker D, Klionsky S, Laskowski MJ. Botany: A record-breaking pollen catapult. Nature. 2005;435(7039):164. doi: 10.1038/435164a. [DOI] [PubMed] [Google Scholar]
- 8.Forterre Y, Skotheim JM, Dumais J, Mahadevan L. How the Venus flytrap snaps. Nature. 2005;433(7024):421–425. doi: 10.1038/nature03185. [DOI] [PubMed] [Google Scholar]
- 9.Vincent O, et al. Ultra-fast underwater suction traps. Proc Biol Sci. 2011;278(1720):2909–2914. doi: 10.1098/rspb.2010.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogel S. Living in a physical world III. Getting up to speed. J Biosci. 2005;30(3):303–312. doi: 10.1007/BF02703667. [DOI] [PubMed] [Google Scholar]
- 11.Juniper BE, Robins RJ, Joel DM. The Carnivorous Plants. Academic; London: 1989. [Google Scholar]
- 12.Iijima T, Sibaoka T. Action potential in the trap lobes of Aldrovanda vesiculosa. Plant Cell Physiol. 1981;22(8):1595–1601. [Google Scholar]
- 13.Di Palma JR, Mohl R, Best W., Jr Action potential and contraction of Dionaea muscipula (Venus flytrap) Science. 1961;133(3456):878–879. doi: 10.1126/science.133.3456.878. [DOI] [PubMed] [Google Scholar]
- 14.Volkov AG, Adesina T, Markin VS, Jovanov E. Kinetics and mechanism of Dionaea muscipula trap closing. Plant Physiol. 2008;146(2):694–702. doi: 10.1104/pp.107.108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poppinga S, et al. Catapulting tentacles in a sticky carnivorous plant. PLoS One. 2012;7(9):e45735. doi: 10.1371/journal.pone.0045735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams SE, Pickard BG. Receptor potentials and action potentials in Drosera tentacles. Planta. 1972;103(3):193–221. doi: 10.1007/BF00386844. [DOI] [PubMed] [Google Scholar]
- 17.Sydenham PH, Findlay GP. The rapid movement of the bladder of Utricularia sp. Aust J Biol Sci. 1973;26:1115–1126. [Google Scholar]
- 18.McPherson S. Pitcher Plants of the Old World. Redfern Natural History; Poole, UK: 2009. [Google Scholar]
- 19.Moran JA. Pitcher dimorphism, prey pomposition and the mechanisms of prey attraction in the pitcher plant Nepenthes rafflesiana in Borneo. J Ecol. 1996;84(4):515–525. [Google Scholar]
- 20.Giusto BD, Grosbois V, Fargeas E, Marshall DJ, Gaume L. Contribution of pitcher fragrance and fluid viscosity to high prey diversity in a Nepenthes carnivorous plant from Borneo. J Biosci. 2008;33(1):121–136. doi: 10.1007/s12038-008-0028-5. [DOI] [PubMed] [Google Scholar]
- 21.Moran JA, Booth WE, Charles JK. Aspects of pitcher morphology and spectral characteristics of six Bornean Nepenthes pitcher plant species: Implications for prey capture. Ann Bot (Lond) 1999;83(5):521–528. [Google Scholar]
- 22.Joel DM, Juniper BE, Dafni A. Ultraviolet patterns in the traps of carnivorous plants. New Phytol. 1985;101(4):585–593. [Google Scholar]
- 23.Merbach MA, Zizka G, Fiala B, Maschwitz U, Booth WE. Patterns of nectar secretion in five Nepenthes species from Brunei Darussalam, Northwest Borneo, and implications for ant-plant relationships. Flora. 2001;196(2):153–160. [Google Scholar]
- 24.Bennett KF, Ellison AM. Nectar, not colour, may lure insects to their death. Biol Lett. 2009;5(4):469–472. doi: 10.1098/rsbl.2009.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bauer U, Willmes C, Federle W. Effect of pitcher age on trapping efficiency and natural prey capture in carnivorous Nepenthes rafflesiana plants. Ann Bot (Lond) 2009;103(8):1219–1226. doi: 10.1093/aob/mcp065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bauer U, Bohn HF, Federle W. Harmless nectar source or deadly trap: Nepenthes pitchers are activated by rain, condensation and nectar. Proc Biol Sci. 2008;275(1632):259–265. doi: 10.1098/rspb.2007.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bohn HF, Federle W. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proc Natl Acad Sci USA. 2004;101(39):14138–14143. doi: 10.1073/pnas.0405885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaume L, Forterre Y. A viscoelastic deadly fluid in carnivorous pitcher plants. PLoS One. 2007;2(11):e1185. doi: 10.1371/journal.pone.0001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bauer U, Grafe TU, Federle W. Evidence for alternative trapping strategies in two forms of the pitcher plant, Nepenthes rafflesiana. J Exp Bot. 2011;62(10):3683–3692. doi: 10.1093/jxb/err082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaume L, et al. How do plant waxes cause flies to slide? Experimental tests of wax-based trapping mechanisms in three pitfall carnivorous plants. Arthropod Struct Dev. 2004;33(1):103–111. doi: 10.1016/j.asd.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Gorb E, et al. Composite structure of the crystalline epicuticular wax layer of the slippery zone in the pitchers of the carnivorous plant Nepenthes alata and its effect on insect attachment. J Exp Biol. 2005;208(Pt 24):4651–4662. doi: 10.1242/jeb.01939. [DOI] [PubMed] [Google Scholar]
- 32.Gaume L, Di Giusto B. Adaptive significance and ontogenetic variability of the waxy zone in Nepenthes rafflesiana. Ann Bot (Lond) 2009;104(7):1281–1291. doi: 10.1093/aob/mcp238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moran JA, Gray LK, Clarke C, Chin L. Capture mechanism in Palaeotropical pitcher plants (Nepenthaceae) is constrained by climate. Ann Bot (Lond) 2013;112(7):1279–1291. doi: 10.1093/aob/mct195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonhomme V, et al. Slippery or sticky? Functional diversity in the trapping strategy of Nepenthes carnivorous plants. New Phytol. 2011;191(2):545–554. doi: 10.1111/j.1469-8137.2011.03696.x. [DOI] [PubMed] [Google Scholar]
- 35.Bauer U, Di Giusto B, Skepper J, Grafe TU, Federle W. With a flick of the lid: A novel trapping mechanism in Nepenthes gracilis pitcher plants. PLoS One. 2012;7(6):e38951. doi: 10.1371/journal.pone.0038951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Federle W, Baumgartner W, Hölldobler B. Biomechanics of ant adhesive pads: Frictional forces are rate- and temperature-dependent. J Exp Biol. 2004;207(Pt 1):67–74. doi: 10.1242/jeb.00716. [DOI] [PubMed] [Google Scholar]
- 37.Bullock JMR, Drechsler P, Federle W. Comparison of smooth and hairy attachment pads in insects: Friction, adhesion and mechanisms for direction-dependence. J Exp Biol. 2008;211(Pt 20):3333–3343. doi: 10.1242/jeb.020941. [DOI] [PubMed] [Google Scholar]
- 38.Vogel S. Living in a physical world II. The bio-ballistics of small projectiles. J Biosci. 2005;30(2):167–175. doi: 10.1007/BF02703696. [DOI] [PubMed] [Google Scholar]
- 39.Vaughn KC, Bowling AJ, Ruel KJ. The mechanism for explosive seed dispersal in Cardamine hirsuta (Brassicaceae) Am J Bot. 2011;98(8):1276–1285. doi: 10.3732/ajb.1000374. [DOI] [PubMed] [Google Scholar]
- 40.Evangelista D, Hotton S, Dumais J. The mechanics of explosive dispersal and self-burial in the seeds of the filaree, Erodium cicutarium (Geraniaceae) J Exp Biol. 2011;214(Pt 4):521–529. doi: 10.1242/jeb.050567. [DOI] [PubMed] [Google Scholar]
- 41.Taylor PE, Card G, House J, Dickinson MH, Flagan RC. High-speed pollen release in the white mulberry tree, Morus alba L. Sex Plant Reprod. 2006;19(1):19–24. [Google Scholar]
- 42.Bauer U, Federle W, Seidel H, Grafe TU, Ioannou CC. How to catch more prey with less effective traps: Explaining the evolution of temporarily inactive traps in carnivorous pitcher plants. Proc Biol Sci. 2015;282(1801):20142675. doi: 10.1098/rspb.2014.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abbott KL. Supercolonies of the invasive yellow crazy ant, Anoplolepis gracilipes, on an oceanic island: Forager activity patterns, density and biomass. Insectes Soc. 2005;52(3):266–273. [Google Scholar]
- 44.Chown SL, Klok CJ, McGeoch MA. Weather to go out: Activity of Bothrometopus brevis (Curculionidae) at Heard Island. Polar Biol. 2004;27(4):217–221. [Google Scholar]
- 45.Juillet JA. Influence of weather on flight activity of parasitic Hymenoptera. Can J Zool. 1964;42(6):1133–1141. [Google Scholar]
- 46.Zhang Q-H, Schlyter F, Chu D, Ma X-Y, Ninomiya Y. Diurnal and seasonal flight activity of males and population dynamics of fall webworm moth, Hyphantria cunea, (Drury) (Lep., Arctiidae) monitored by pheromone traps. J Appl Entomol. 1998;122(1–5):523–532. [Google Scholar]
- 47.Jeffree CE. The cuticle, epicuticular waxes and trichomes of plants, with reference to their structure, functions and evolution. In: Juniper BE, Southwood TRE, editors. Insects and the Plant Surface. Edward Arnold; London: 1986. pp. 23–64. [Google Scholar]
- 48.Eigenbrode SD. The effects of plant epicuticular waxy blooms on attachment and effectiveness of predatory insects. Arthropod Struct Dev. 2004;33(1):91–102. doi: 10.1016/j.asd.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 49.Eigenbrode SD, Espelie KE. Effects of plant epicuticular lipids on insect herbivores. Annu Rev Entomol. 1995;40(1):171–194. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.