Figure 8.
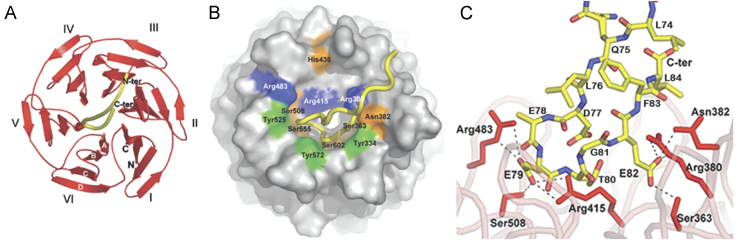
Structures of the Kelch domain of human Keap1 bound to an Nrf2 peptide. (A) A top-down view showing the six-bladed β-propeller structure in red ribbon and the peptide as a yellow tube. Each blade of the β-propeller is numbered I–VI. Both the N- and C-termini of the domain are located in blade I and are labeled N and C, respectively. The four β-strands found in each blade are designated A–D as shown in white font on blade VI. (B) A surface representation of the Kelch propeller (gray) and peptide (yellow tube). Selected residues are shown in blue (basic), orange (polar) and green (apolar). (C) Charge–charge and H-bonding interactions between the side chain atoms of the Nrf2 peptide and residues in the Kelch domain. Not shown are 5 H-bond interactions between the peptide backbone atoms and residues in the Kelch domain (reproduced with permission from reference108).