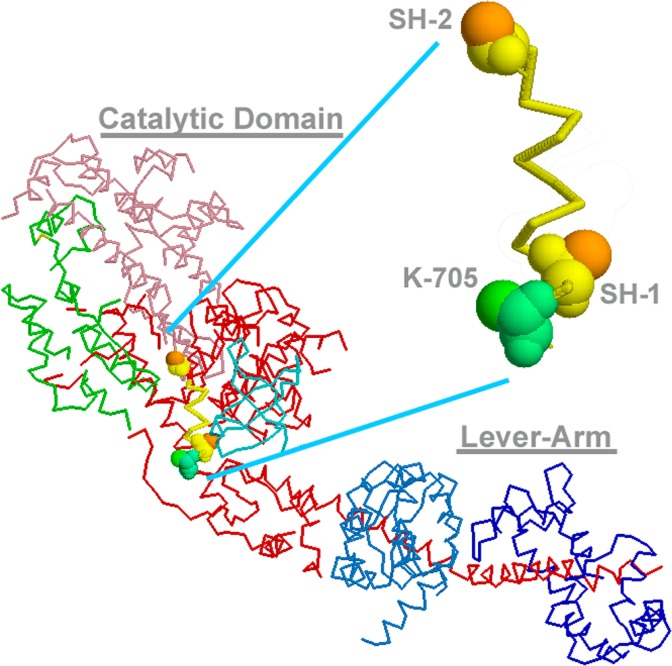
Figure 2.
Crystal structure of scallop myosin S1 under nucleotide-free rigor state (1DFK) and the 3-D spatial arrangement of SH1, SH2 (Cys-703 and Cys-693, in the scallop’s sequence) and Lys-705 along SH1-helix. SH1-helix portion is enlarged to indicate that two thiols are pointing to the opposite directions, where disulfide formation is impossible. Lys-705 is protruding to the same side as SH2 and they were actually cross-inked by p-PDM30,37 to give 1L20 structure. The lever-arm might flip to the other side, when SH1-helix is disrupted so that two thiols form disulfide.