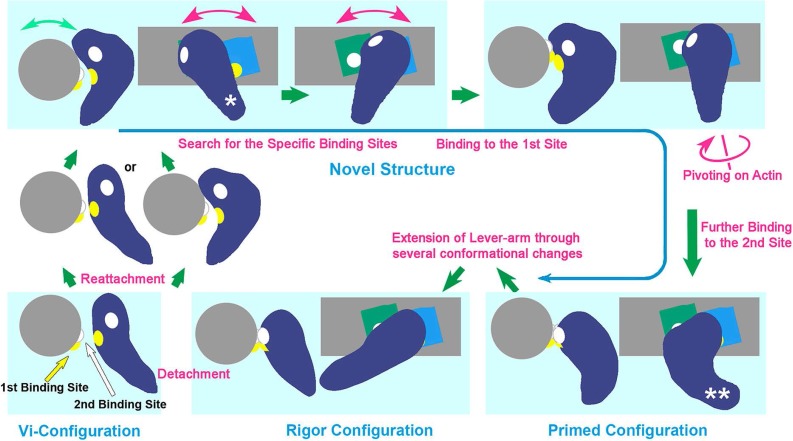
Figure 4.
Schematic drawing of revised cross-bridge-cycle of skeletal actomyosin including the new conformer (taken from Fig. 8 of the reference 26). The cycle starts from the dissociation of rigor myosin from actin by the binding of ATP, which eventually re-associates with actin through several conformational states mostly involving the new configuration. The structural states placed in light-bluish panels are presented as the side- and the barbed-end views of the actomyosin complex. Violet; myosin head, blue and green; actin-monomers along actin-filament (gray). There, the behavior of two pairs of actomyosin interaction sites (presented by yellow and white patches in the reaction sequence), are indicated according to the time-sequence of the structural change from conventionally-bent ADP/Pi-structure (similar to the ADP/Vi-bound form) right after dissociation from actin, to the final rigor structure [N.B.; The third actin-binding site is located close to the yellow patch and might concomitantly operate with the second site to start lever-arm swinging]. Hence, the new conformer plays a crucial role as the reaction intermediate, throughout those states. The configurations marked by a single and double asterisk in the drawing correspond to the views (d) and (c) in Figure 3, respectively. See text for details.