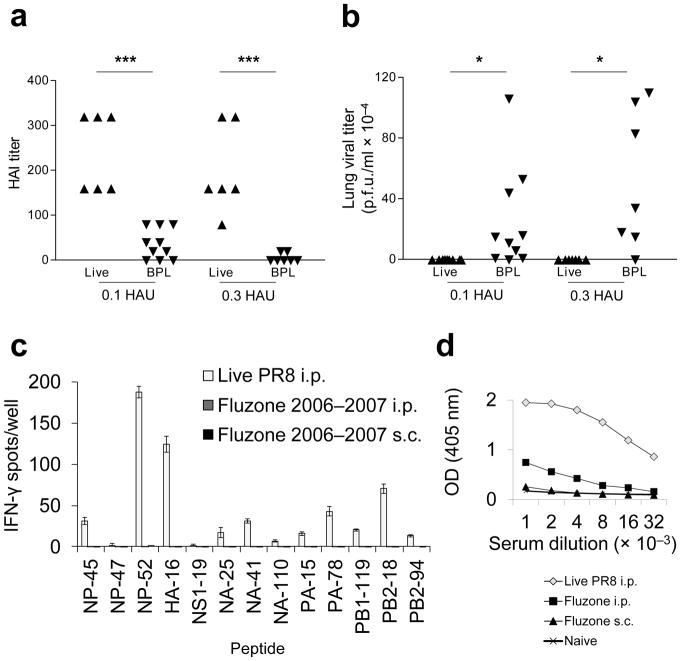
Figure 2.
Vaccination with inactivated PR8 induces low levels of influenza-specific neutralizing antibodies, which poorly control virus replication. The licensed split subunit vaccine similarly displays poor CD4+ T cell and B cell immunogenicitty. (a) Mice were primed i.m with 0.1 HAU live PR8 (▲) (n = 7), 0.1 HAU BPL PR8 (▼) (n = 10), 0.3 HAU live PR8 (▲) (n = 7), or 0.3 HAU BPL PR8 (▼) (n = 7) and serum HAI antibody titers were quantified on d26 (left graph). (b)They were then challenged i.n with 20 HAU live homologous PR8. Live virus was titered by plaque assay from lung homogenates 3 days post immunization (d.p.i.). (c) B6 mice were primed i.p. with 4 HAU live PR8 (white bars), 22.5 μg H1 Fluzone 2006–2007 formula i.p. (dark gray bars, not visible), or 22.5 μg H1 Fluzone 2006–2007 formula s.c. (black bars, not visible). This is higher than the dose administered to humans (15 μg HA each). 12 days later, cytokine ELISpot assays were performed for detection of IFN-γ-secreting CD4+ T cells in response to individual influenza peptides. (d) Serum IgG titer to PR8 was determined by ELISA. Sera were collected on d12 from the same mouse groups - live PR8 i.p. (◇), Fluzone i.p. (■), Fluzone s.c. (▲) - inoculated in panel (c) and sera from naive (×) mice were used as controls. (c) (representative of two independent experiments performed in triplicate) (d) (representative of two independent experiments performed in duplicate, error bars covered by symbols). Data are presented as mean ± s.d. Background (mean DMSO + 2s.d.) was subtracted from the experimental group results. Statistical significance was tested (for a and b) by one-tailed Student’s t-test and (for d) P < 0.0001 by two-way ANOVA and post-hoc pairwise comparisons with Bonferroni correction.***P < 0.005, * P < 0.05.