Figure 8. The mechanical switch model for attachment-sensitive SAC signaling.
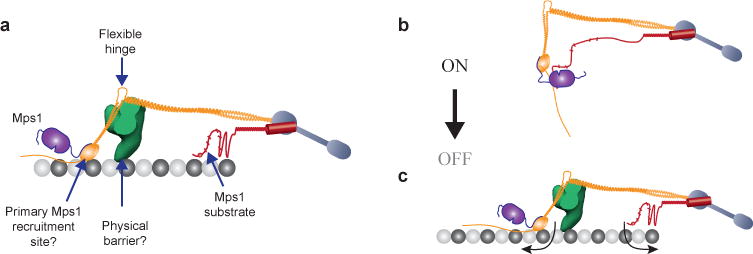
(a) Key features of the metaphase architecture of the yeast kinetochore-microtubule attachment (when SAC signaling is off), and their potential roles in executing attachment-dependent SAC signaling. Please note that the schematic displays a possible organization of Spc105 phosphodomain; the actual organization remains unknown. The microtubule-binding activity of this domain is not highlighted.
(b–c) The protein architecture of the kinetochore encodes a mechanical switch that controls SAC signaling. The CH-domain of Ndc80 and the phosphodomain of Spc105 act as the two terminals of this switch. It is in the ‘on’ state in unattached kinetochores, because the inherent flexibilities in the Ndc80 complex and Spc105 position the two terminals in close proximity. This allows Mps1 to phosphorylate Spc105 and initiate SAC signaling. Microtubule attachment toggles this switch to its ‘off’ state by physically separating the two terminals (arrows). Mps1 can no longer phosphorylate Spc105. The Dam1 complex, which is recruited to the kinetochore only after end-on microtubule attachment is established, may create a barrier that further hinders the interaction between Mps1 and Spc105. This leads to SAC silencing.
