Abstract
Purpose
Adiponectin is expressed in adipose tissue, and is affected by smoking, obesity, and genetic factors, such as CDH13 polymorphism, contributing to the development of coronary vascular diseases (CVDs).
Materials and Methods
We investigated the effect of genetic variations of CDH13 (rs3865188) on blood chemistry and adiponectin levels in 345 CVD patients undergoing statin-free or statin treatment.
Results
Genetic variation in CDH13 was significantly correlated with several clinical factors, including adiponectin, diastolic blood pressure, triglyceride (TG), and insulin levels. Subjects with the T allele (mutant form) had significantly lower adiponectin levels than those with the A allele. Total cholesterol (TC), low-density lipoprotein cholesterol (LDLc), TG/high-density lipoprotein cholesterol (HDLc) ratio, and HDL3b subtype were markedly decreased in statin treated subjects regardless of having the A or T allele. TG and TG/HDL in the statin-free group with TT genotype of the rs3865188 was higher than in the others but they were not different in the statin-treated subjects. We observed a significant difference in adiponectin levels between patients with the A and T alleles in the statin-free group; meanwhile, no difference in adiponectin levels was noted in the statin group. Plasma levels of other cytokines, leptin, visfatin, interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α), were not different among the CDH13 genotypes according to statin administration. Body mass index (BMI), TG, insulin, HDL3b, and TG/HDL ratio showed negative correlations with adiponectin levels.
Conclusion
Plasma adiponectin levels and TG/HDL ratio were significantly different according to variants of CDH13 and statin administration in Korean patients with CVD.
Keywords: Adiponectin, CDH13, statin, TG/HDL, HDL3b
INTRODUCTION
Some adipokines are produced in adipose tissue, while others are partially secreted by infiltrating macrophages and stromal cells.1 Among adipokines, adiponectin is only expressed by adipocytes, and stromal vascular cells of adipose tissue. Adiponectin is known to increase insulin-stimulated glucose uptake by enhancing Akt phosphorylation. Plasma adiponectin levels have been found to be correlated with body mass index (BMI), type 2 diabetes mellitus (T2DM), and coronary vascular disease (CVD). Additionally, decreased expression, secretion, and circulating levels of adiponectin have been reported in obese populations.1,2,3 Adiponectin treatment reduces the expression of endotoxin-induced pro-inflammatory cytokines and increases anti-inflammatory interleukin-10 (IL-10) expression in monocytes and macrophages. Exogenous administration of adiponectin also decreases allergic airway inflammation in mice.4
In spite of its anti-inflammatory and anti-atherogenic effects, adiponectin is potentially associated with adverse clinical outcomes, such as mortality. As plasma adiponectin levels are strongly influenced by single nucleotide polymorphisms (SNPs) of the gene encoding T-cadherin [the cadherin 13 preprotein (CDH13)], several studies had been conducted to examine the possible associations between CDH13 genotype and plasma adiponectin levels.5 CDH13, a 95 kd glycoprotein, is an atypical member of the cadherin family of cell adhesion molecules.4 Unlike classical cadherins, it has low adhesive characters and is attached to the cell membrane via a glycosylphosphatidyli-nositol anchor, as it lacks both transmembrane and cytoplasmic domains.6 CDH13 functions as a negative regulating molecule during the development of the nervous system and is involved in migratory processes, tumorigenesis, and angiogenesis.7,8 Moreover, CDH13 is involved in endoplasmic reticulum (ER) stress responses. Several studies suggested CDH13 is upregulated in endothelial cells after induction of ER stress and protect these cells from apoptosis in reaction to the proapoptotic response.9 Another report observed that GRP78, a molecular chaperone similar to CDH13, was involved in pro-survival responses to ER stress, as a signaling partner to CDH13, along to the surface of endothelial cells.10 Recently, several CDH13 SNPs, such as rs3865188, have been reported to be determinants of blood adiponectin levels in Asians. The association of rs11646213 in CDH13 and blood pressure phenotype has also been investigated in European cohorts. Another SNP, rs4783244, was also shown to be associated with metabolic syndrome phenotype in Taiwanese.11 However, the association between CDH13 variations and cardiometabolic disease is not completely clear.
Statins [or 3-hydroxy-3-methyl-glutaryl (HMG)-CoA reductase inhibitors] are known to be effective in reducing risk of cardiovascular events in patients with CVD by lowering cholesterol synthesis.12 Several reports have demonstrated that statin shows pleiotropic effects in modulating endothelial function, stable angina pectoris, inflammation, and thrombosis.13,14 However, only the impact of APOE on statin treatment efficacy has been reported, although the APOE4 allele is associated with a poor response to statin treatment.15 Therefore, in this study, we aimed to examin the effect of CDH13 rs3865188 on adiponectin levels and lipid profiles in 345 statin-free or statin-treated outpatients enrolled with the Cardiovascular Center at Severance Hospital in Seoul, Korea.
MATERIALS AND METHODS
Study population
A total of 345 outpatients were recruited from the Cardiovascular Genome Center Registry from the Yonsei University College of Medicine, supported by The Ministry of Health and Welfare. Briefly, consecutive subjects who visited the Cardiovascular Center at Severance Hospital in Seoul, Korea from July 2008 to December 2009 were prospectively screened for dyslipidemia [triglyceride (TG) >150 mg/dL; high-density lipoprotein-cholesterol (HDLc) <40 mg/dL for men, HDL <50 mg/dL for women]. Subjects who exhibited positive results for dyslipidemia on three separate visits and patients who had taken anti-hyperlipidemic medications such as statins were also included. For further analysis of the association between genetic variants and other metabolic traits, the study population was divided into two groups: a statin-free group and a statin group. We analyzed the effects of CDH13 rs3865188 on blood chemistry with statin groups compared to the statin free groups. This study was conducted according to the guidelines of the Declaration of Helsinki, and was approved by the Institutional Review Board of Yonsei University Medical Center (IRB#: 4-2001-0039). Written informed consent was obtained from all study participants.
Clinical data and blood samples
On the day of enrollment, clinical data including demographic variables and medical history were recorded. We calculated BMI as a ratio of weight (kg) divided by the height (m2). Blood lipid profiles [total cholesterol (TC), low-density lipoprotein-cholesterol (LDLc), HDLc, TG, and HDL subtypes], fasting blood sugar (FBS), insulin, enzymes related to HDL metabolism [lecithin cholesterol acyltransferase (LCAT) and cholesteryl ester transfer protein (CETP)], and adipocytokines [adiponectin, leptin, monocyte chemoattractant protein-1 (MCP-1), visfatin, IL-6, tumor necrosis factor-α (TNF-α)] were obtained from serum or plasma collected after an overnight fast and kept at -80℃ until further analysis. FBS, TC, and TG were measured using a Hitachi-7600 analyzer (Hitachi Ltd., Tokyo, Japan), and LDLc was calculated by Friedewald equation. The 600 µL of fresh serum, kept at 4℃ for less than 1 day, used for HDL subclasses after HDL fraction was isolated by new micro-ultracentrifugation at 1.063<d<1.21 g/mL (Hitachi CS150GXL, CS140AT fixed angle rotor, Tokyo, Japan), and separation of HDL subfractions were conducted as previously reported.16 All cytokines, as well as LCAT and CETP, were measured by enzyme immunoassay using an ELISA kit (Diichi Pure Chemicals, Tokyo, Japan). Serum adiponectin and insulin levels were measured by an enzyme-linked immunosorbent assay (Mesdia Co. Ltd., Seoul, Korea).17 The intra- and inter-assay variances for adiponectin were 6.3-7.4% and 4.5-8.6%, respectively.
Sequencing and genotyping
PCR primers were designed to independently amplify CDH13 fragments. Primer sequences are available on request. PCR products were purified and then sequenced using a BigDye Terminator Cycle Sequencing Kit (version 3.1, ABI, Foster City, CA, USA) and an ABI 3730×1 automated sequencer (Applied Biosystems, Foster City, CA, USA). The sequencing primers were the same as those used for PCR amplification. SNPs identified in the CDH13 gene by whole gene sequencing were genotyped. Genomic DNA was extracted from 5 mL of peripheral venous blood using a commercially available isolation kit (QuickGene SP Kit DNA whole blood, Fujifilm, Tokyo, Japan). Genotyping was performed using the TaqMan fluorogenic 5' nuclease assay (ABI).
Statistical analysis
Group differences for categorical variables were assessed by chi-square test, and continuous variables were examined by Student's t-test. The association between genotype and adiponectin level was evaluated using odds ratios (ORs) and 95% confidence intervals (CIs) from chi-square tests and logistic regression analyses. The results are presented as mean±standard error range. p-values for each genotype and allele were evaluated by χ2 analysis and Fisher's exact test. A p-value with three decimal places was taken to indicate statistically significant differences between statin-free and statin-treated subjects of the study. Associations between clinical variables, such as age, BMI, and TC, were analyzed by Pearson correlation test. All analyses were performed using SPSS software, version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Characteristics of the study population according to genotype and alleles of rs3865188
Characteristics of the population are shown in Table 1. The 345 subjects recruited were aged 62.56±10.0 years, and 67.3% (n=233) of the total population were men. Subjects with the CDH13 SNP showed significant differences in several clinical factors, including adiponectin level; diastolic blood pressure (DBP); and levels of FBS, insulin, and TG (Table 1). Subjects with the T allele (mutant form) had significantly lower adiponectin and insulin levels and significantly higher plasma TG than those with the A allele (wild-type form) (p<0.05). In contrast, alleles of the CDH13 gene showed a divergent trend when compared with the genotype. There were significant differences in FBS level and DBP between heterozygous subjects with the AT allele and homozygous subjects with an AA or TT allele, which may have contributed to the non-significant differences observed in the FBS level and DBP between A and T alleles. Other blood chemistry data, such as lipid profiles with HDL subtypes or inflammatory cytokines, did not differ by CDH13 genotype or allele types.
Table 1. Anthropometric and Clinical Characteristics of Cardiovascular Disease Patients According to Allele and Genotype of CDH13 Gene SNP (rs3865188).
| Variables | Total (n=345) | Genotype | Allele | |||||
|---|---|---|---|---|---|---|---|---|
| AA (n=170) | AT (n=128) | TT (n=36) | p value* | A (n=469) | T (n=201) | p value* | ||
| Adiponectin | 5.81±3.70 | 006.25±3.76b | 005.79±3.66b | 003.97±3.29a | 0.046 | 6.13±3.72 | 5.08±3.60 | 0.016 |
| Age (yr) | 62.56±10.00 | 63.31±10.07 | 61.91±9.53 | 62.92±10.38 | 0.481 | 62.93±9.92 | 62.28±9.80 | 0.451 |
| BMI (kg/m2) | 25.09±3.25 | 24.91±3.23 | 25.40±3.49 | 24.78±2.44 | 0.371 | 25.04±3.31 | 25.17±3.15 | 0.618 |
| SBP (mm Hg) | 116.73±13.49 | 116.90±12.27 | 115.29±14.15 | 118.33±12.98 | 0.382 | 116.47±12.79 | 116.40±13.75 | 0.929 |
| DBP (mm Hg) | 71.39±8.30 | 72.09±7.70 | 69.96±7.81 | 72.22±7.96 | 0.052 | 71.52±7.77 | 70.79±7.90 | 0.717 |
| TC (mg/dL) | 150.50±38.74 | 147.64±37.80 | 155.60±39.89 | 146.88±36.84 | 0.176 | 149.82±38.47 | 152.46±38.87 | 0.419 |
| TG (mg/dL) | 142.71±81.29 | 134.96±78.14 | 147.77±80.39 | 158.13±90.92 | 0.184 | 138.46±78.80 | 151.50±84.00 | 0.055 |
| HDLc (mg/dL) | 39.96±9.69 | 40.47±9.66 | 39.49±9.41 | 39.63±10.38 | 0.668 | 40.20±9.58 | 39.54±9.72 | 0.411 |
| LDLc (mg/dL) | 85.72±31.51 | 83.04±30.57 | 90.88±32.31 | 81.30±31.18 | 0.067 | 85.18±31.19 | 87.43±32.09 | 0.403 |
| TG/HDL | 3.94±2.97 | 3.67±2.84 | 4.10±2.96 | 4.48±3.39 | 0.226 | 3.79±2.87 | 4.24±3.11 | 0.070 |
| Insulin | 10.91±11.26 | 12.36±14.19 | 9.87±8.12 | 8.70±4.84 | 0.088 | 11.66±12.80 | 9.45±7.11 | 0.006 |
| HOMA-IR | 3.63±5.50 | 3.96±7.02 | 3.57±4.01 | 2.41±1.37 | 0.328 | 3.85±6.31 | 3.15±3.35 | 0.069 |
| FBS | 125.00±52.84 | 118.49±42.13 | 134.00±65.52 | 116.13±41.93 | 0.026 | 122.73±50.00 | 127.57±58.58 | 0.316 |
| CRP | 0.33±0.78 | 0.26±0.60 | 0.49±1.07 | 0.23±0.41 | 0.344 | 0.31±0.75 | 0.39±0.87 | 0.550 |
| Leptin | 29.55±30.05 | 27.25±29.86 | 35.36±34.14 | 22.61±12.87 | 0.145 | 29.28±31.05 | 30.36±28.38 | 0.751 |
| MCP-1 | 44.81±38.40 | 40.08±34.28 | 53.97±44.53 | 42.03±36.00 | 0.084 | 43.55±37.44 | 49.29±41.46 | 0.219 |
| Visfatin | 1.87±0.12 | 1.86±0.12 | 1.87±0.12 | 1.89±0.13 | 0.489 | 1.86±0.12 | 1.88±0.12 | 0.208 |
| IL-6 | 37.48±56.05 | 40.40±58.46 | 38.56±60.67 | 24.70±26.81 | 0.530 | 39.94±58.78 | 33.12±50.40 | 0.312 |
| TNF-α | 26.95±32.07 | 29.68±39.62 | 23.30±19.95 | 22.92±15.81 | 0.406 | 28.09±35.71 | 23.15±18.29 | 0.087 |
| BUN | 16.38±4.77 | 16.33±4.55 | 16.13±4.54 | 17.63±6.39 | 0.344 | 16.28±4.53 | 16.65±5.26 | 0.414 |
| Uric acid | 5.37±1.45 | 5.31±1.44 | 5.37±1.50 | 5.59±1.31 | 0.699 | 5.33±1.45 | 5.45±1.43 | 0.418 |
| HDL2b | 31.79±3.08 | 31.96±3.01 | 31.52±3.08 | 31.89±3.23 | 0.509 | 31.83±3.03 | 31.65±3.12 | 0.505 |
| HDL2a | 22.27±1.57 | 22.19±1.72 | 22.39±1.39 | 22.15±1.58 | 0.550 | 22.24±1.63 | 22.30±1.45 | 0.679 |
| HDL3a | 18.56±1.70 | 18.45±1.71 | 18.68±1.62 | 18.51±1.71 | 0.548 | 18.52±1.68 | 18.62±1.64 | 0.495 |
| HDL3b | 12.73±1.51 | 12.70±1.47 | 12.81±1.55 | 12.63±1.57 | 0.787 | 12.73±1.49 | 12.75±1.55 | 0.919 |
| HDL3c | 14.55±2.33 | 14.54±2.46 | 14.58±2.20 | 14.71±2.45 | 0.932 | 14.55±2.38 | 14.63±2.28 | 0.719 |
| LCAT mass | 8.72±2.76 | 8.62±2.84 | 8.80±2.83 | 8.98±2.36 | 0.809 | 8.67±2.83 | 8.87±2.64 | 0.491 |
| CETP mass | 1.62±0.67 | 1.55±0.56 | 1.67±0.68 | 1.63±0.74 | 0.466 | 1.58±0.59 | 1.66±0.70 | 0.312 |
| LCAT/CETP | 6.13±2.80 | 6.15±2.78 | 6.06±2.76 | 6.47±3.18 | 0.821 | 6.12±2.77 | 6.21±2.91 | 0.782 |
SNP, single nucleotide polymorphism; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; TG, triglyceride; HDLc, high-density lipoprotein cholesterol; LDLc, low-density lipoprotein cholesterol; FBS, fasting blood sugar; CRP, C-reactive protein; MCP-1, monocyte chemoattractant protein-1; IL-6, interleukin 6; TNF-α, tumor necrosis factor-α; HOMA-IR, homeostasis model assessment of insulin resistance.
Values with different alphabets are significantly different among the groups by ANOVA at p<0.005.
*p value was estimated by ANOVA and t-test.
CDH13 variants and statin treatment on lipid risk factors
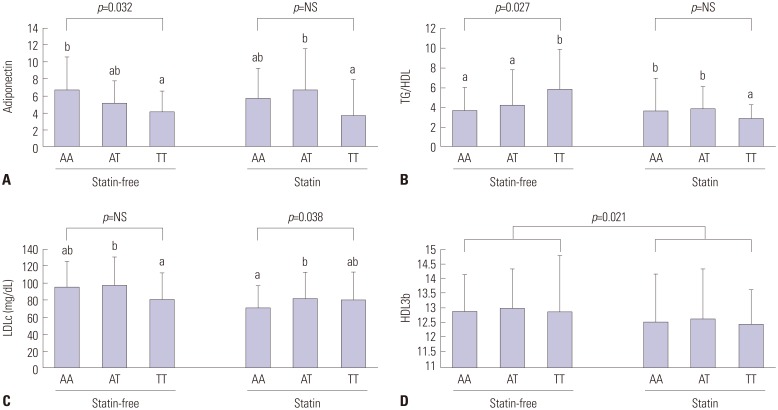
One SNP (rs3865188) was significantly associated with adiponectin level. In the additive model, adiponectin level was not associated with a decreased risk of dyslipidemia (OR=0.732, 95% CI, 0.349-1.545, p=0.406) (data not shown). Subjects with the A allele had higher adiponectin levels than those with the T allele in the statin-free group, but there were no differences in statin groups (Table 2). There was also a difference in TG/HDL levels between subjects with the T allele and those with the A allele in the statin-free group. Moreover, there was no difference in TG/HDL and adiponectin levels in the statin group. The subjects with a minor T allele of rs3865188 had higher LDLc and MCP-1 levels, whereas those with rs3865188 had lower insulin levels in the statin group (p=0.003). Statin treatment strongly decreased TC, LDLc, TG/HDL ratio, and HDL3b subfractions regardless of having the A or T allele, respectively. In the statin-free group, subjects with the TT genotype of the rs3865188 SNP had lower adiponectin levels (p=0.032) and higher TG levels (p=0.025) (Fig. l). In the statin group, however, the TT genotype was strongly associated with reductions in TG levels and in TG/HDL. Plasma adiponectin levels were significantly increased for individuals with the AT genotype and those who underwent statin treatment.
Table 2. Anthropometric and Clinical Characteristics of Cardiovascular Disease Patients with CDH13 SNP (rs3865188) Alleles According to Statin Administration.
| Variables | Statin-free group (n=177) | Statin group (n=168) | p value* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | A | T | p value† | Total | A | T | p value† | ||
| Adiponectin | 5.83±3.45 | 6.25±3.70b | 4.78±2.56a | 0.007 | 5.79±4.00 | 5.99±3.77ab | 5.45±4.61ab | 0.441 | 0.073 |
| Age (yr) | 62.31±10.13 | 62.92±9.72 | 62.22±10.15 | 0.543 | 62.87±9.84 | 62.97±10.14 | 62.43±9.44 | 0.654 | 0.898 |
| BMI (kg/m2) | 24.96±.3.07 | 24.77±3.10 | 25.21±2.91 | 0.228 | 25.23±3.41 | 25.31±3.48 | 25.15±3.40 | 0.709 | 0.341 |
| SBP (mm Hg) | 116.88±13.74 | 116.02±12.26 | 116.81±14.59 | 0.606 | 116.52±13.21 | 116.87±13.32 | 115.82±12.78 | 0.516 | 0.855 |
| DBP (mm Hg) | 71.78±8.26 | 71.61±7.33 | 71.32±7.23 | 0.733 | 74.66±48.40 | 74.09±41.42 | 76.79±64.49 | 0.654 | 0.586 |
| TC (mg/dL) | 161.62±38.52 | 161.57±38.12b | 161.81±38.06b | 0.958 | 138.77±35.47 | 137.86±35.05a | 141.92±37.22a | 0.346 | <0.001 |
| TG (mg/dL) | 149.81±85.70 | 140.85±76.21a | 164.83±97.75b | 0.026 | 135.23±75.90 | 136.04±81.44a | 136.46±62.29a | 0.936 | 0.017 |
| HDLc (mg/dL) | 39.37±10.13 | 39.94±9.92 | 38.83±10.54 | 0.345 | 40.58±9.19 | 40.46±9.24 | 40.35±8.69 | 0.901 | 0.540 |
| LDLc (mg/dL) | 95.23±31.61 | 96.46±30.80c | 92.46±32.69c | 0.277 | 75.71±28.21 | 73.71±27.19a | 81.77±30.57b | 0.020 | <0.001 |
| TG/HDL | 4.23±3.15 | 3.87±2.71a | 4.81±3.78b | 0.022 | 3.64±2.73 | 3.71±3.03a | 3.59±1.93a | 0.745 | 0.007 |
| Insulin | 10.78±11.69 | 11.24±13.22ab | 9.86±8.10ab | 0.328 | 11.06±10.82 | 12.09±12.36b | 8.99±5.80a | 0.003 | 0.114 |
| HOMA-IR | 3.80±6.84 | 4.03±8.00 | 3.32±3.72 | 0.392 | 3.44±3.54 | 3.66±3.80 | 2.96±2.88 | 0.076 | 0.418 |
| FBS | 125.16±54.81 | 123.63±53.75 | 127.33±58.63 | 0.569 | 124.84±50.86 | 121.81±45.98 | 127.84±58.84 | 0.388 | 0.730 |
| CRP | 0.31±0.57 | 0.29±0.55 | 0.38±0.62 | 0.435 | 0.36±1.03 | 0.35±0.98 | 0.41±1.20 | 0.845 | 0.905 |
| Leptin | 30.66±31.88 | 31.36±34.95 | 29.66±24.10 | 0.738 | 28.17±27.75 | 26.72±25.36 | 31.24±33.29 | 0.346 | 0.668 |
| MCP-1 | 44.76±43.61 | 45.94±43.76ab | 43.29±44.42a | 0.703 | 44.88±31.05 | 40.61±27.63a | 56.89±36.47b | 0.011 | 0.134 |
| Visfatin | 1.87±0.11 | 1.88±0.12 | 1.87±0.10 | 0.940 | 1.86±0.13 | 1.85±0.12 | 1.89±0.15 | 0.067 | 0.148 |
| IL-6 | 38.30±56.34 | 38.90±56.35 | 38.01±57.96 | 0.922 | 36.47±56.03 | 41.23±61.89 | 26.93±38.53 | 0.090 | 0.569 |
| TNF-α | 29.54±39.36 | 31.50±43.89 | 22.44±20.71 | 0.053 | 23.74±19.40 | 23.88±21.22 | 24.05±14.84 | 0.980 | 0.140 |
| BUN | 16.10±4.56 | 15.90±4.22 | 16.68±5.30 | 0.183 | 16.72±4.99 | 16.72±4.86 | 16.61±5.24 | 0.874 | 0.327 |
| Uric acid | 5.31±1.51 | 5.24±1.52 | 5.38±1.46 | 0.530 | 5.43±1.39 | 5.41±1.38 | 5.51±1.40 | 0.598 | 0.567 |
| HDL2b | 31.50±2.94 | 32.06±3.25 | 32.03±3.12 | 0.393 | 32.11±3.21 | 32.06±3.25 | 32.03±3.12 | 0.944 | 0.173 |
| HDL2a | 22.20±1.42 | 22.33±1.86 | 22.42±1.42 | 0.808 | 22.34±1.73 | 22.33±1.86 | 22.42±1.42 | 0.715 | 0.512 |
| HDL3a | 18.59±1.51 | 18.52±1.93 | 18.61±1.79 | 0.550 | 18.52±1.89 | 18.52±1.93 | 18.61±1.79 | 0.694 | 0.925 |
| HDL3b | 12.92±1.37 | 12.54±1.67 | 12.53±1.53 | 0.897 | 12.53±1.63 | 12.54±1.67 | 12.53±1.53 | 0.969 | 0.021 |
| HDL3c | 14.76±2.16 | 14.33±2.61 | 14.33±2.30 | 0.622 | 14.33±2.50 | 14.33±2.61 | 14.33±2.30 | 0.996 | 0.116 |
| LCAT mass | 8.71±2.78 | 8.66±2.83 | 8.96±2.73 | 0.464 | 8.73±2.74 | 8.68±2.84 | 8.77±2.57 | 0.819 | 0.890 |
| CETP mass | 1.64±0.75 | 1.62±0.62 | 1.62±0.76 | 0.990 | 1.59±0.58 | 1.55±0.57 | 1.69±0.63 | 0.111 | 0.488 |
| LCAT/CETP | 6.14±2.89 | 5.98±2.76 | 6.58±3.10 | 0.164 | 6.12±2.72 | 6.26±2.77 | 5.83±2.67 | 0.283 | 0.363 |
SNP, single nucleotide polymorphism; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; TG, triglyceride; HDLc, high-density lipoprotein cholesterol; LDLc, low-density lipoprotein cholesterol; FBS, fasting blood sugar; CRP, C-reactive protein; MCP-1, monocyte chemoattractant protein-1; IL-6, interleukin 6; TNF-α, tumor necrosis factor-α; HOMA-IR, homeostasis model assessment of insulin resistance.
Values with different alphabets are significantly different among the groups by ANOVA at p<0.05.
*p value was estimated by ANOVA, †p value was estimated by t-test.
Fig. 1. Plasma levels of adiponectin (A), TG/HDL (B), LDLc (C), HDL3b (D) subtypes according to CDH13 genotypes whether the stain was taken or not. Values with different alphabets are significantly different among the CHD13 gene genotypes. TG, triglyceride; HDL, high-density lipoprotein; LDLc, low-density lipoprotein cholesterol.
Trends in inflammation markers in subjects who underwent statin treatment
Subjects in the statin group with the TT genotype had higher MCP-1 levels than patients in the statin-free group with the same genotype (57.92±40.90 vs. 26.14±22.39). Statin treatment did not effect reductions in MCP-1 among persons with the AT genotype (Fig. 1). In terms of allele distribution, there was a significant difference in MCP-1 levels in subjects with the T allele, compared to those in subjects with the A allele, in the statin group (p=0.009) (Table 2). On the other hand, individuals with the AT genotype in the statin group had higher leptin levels than wild type patients and mutant homozygous patients. Plasma levels for other cytokines, leptin, visfatin, IL-6, and TNF-α did not differ according to CDH13 genotype or statin administration. TNF-α levels were lower for the T allele than the A allele (Table 2).
Risk factors related to adiponectin level
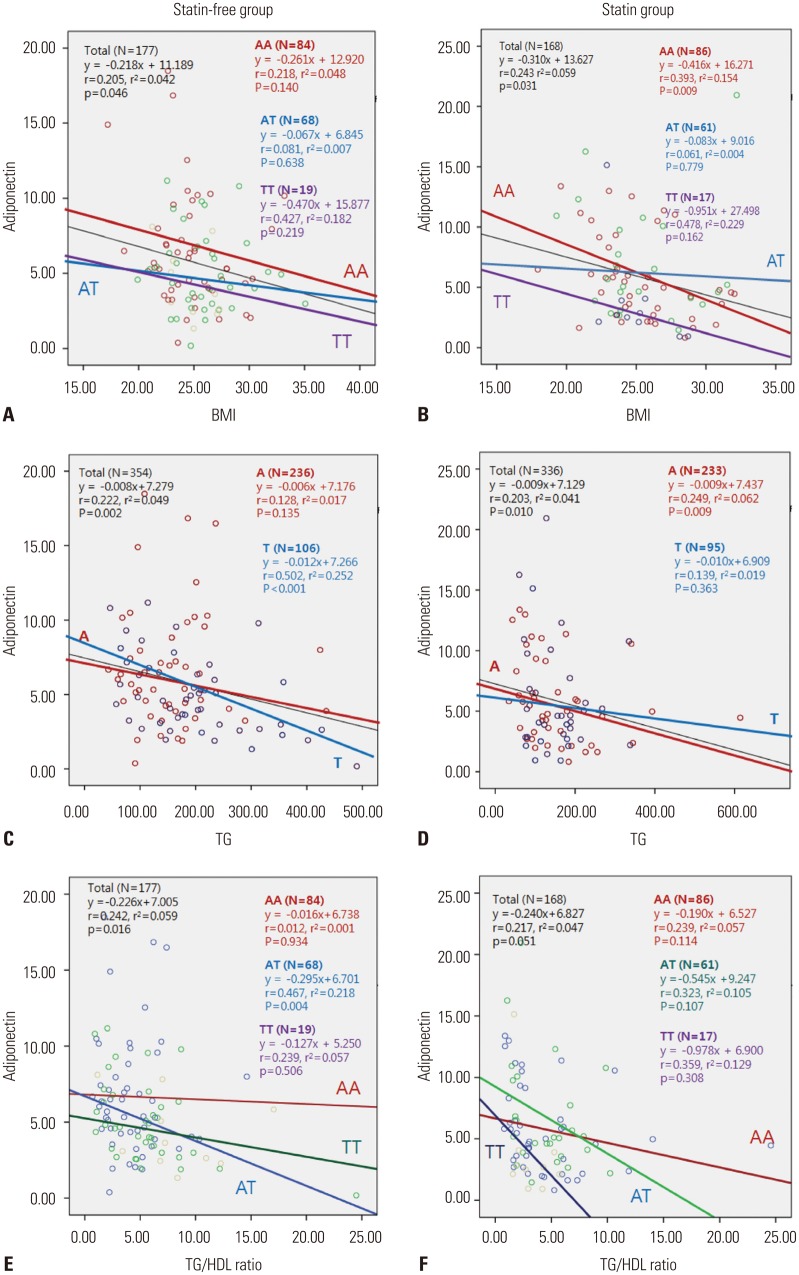
Parametric Pearson correlation analysis of the data revealed that the adiponectin level is significantly positively correlated with sex (r=0.339, p<0.001), HDL level (r=0.353, p<0.001), and HDL2b level (r=0.367, p<0.001). Meanwhile, TG level (r=-0.210, p=0.005), BMI (r=-0.224, p=0.003), insulin level (r=-0.252, p=0.001), HDL3b (r=-0.325, p<0.001), and TG/HDL ratio (r=-0.227, p=0.002) showed negative correlations (data not shown). In the statin-free and statin groups, linear regression analysis was performed to estimate the relative contributions of clinical variables, such as BMI, TG level, insulin level, and HDL2b fraction, to the inter-individual variations in adiponectin level. Therein, TG level was associated with adiponectin level in statin-free individuals with the T allele, not the A allele. In the statin group, there was no association between TG and adiponectin levels in individuals with the T allele (Fig. 2). Plasma adiponectin level was negatively associated with TG/HDL ratio in patients with the AT genotype in the statin-free group. However, the effect of the AT genotype was not observed upon statin treatment.
Fig. 2. Correlation of adiponectin and clinical variables according to genotype distribution of rs3865188. Body mass index (BMI) was negatively correlated with adiponectin in only the AA genotype (p=0.009) in contrast to the TT genotype in statin group; meanwhile, AT genotype can be offset by TT genotype, compared to AA and TT genotype (A and B). Plasma adiponectin was related with TG in A allele of patients taking statin (C and D), and it was related with TG/HDL ratio in the AT genotype in statin-free group. However, with statin treatment, the effect of AT genotype disappeared (E and F). TG, triglyceride; HDL, high-density lipoprotein.
DISCUSSION
This study is the first to suggest a significant association between SNPs located within CDH13 and statin treatment on plasma adiponectin levels and lipid profiles in Korean cardiovascular patients with dyslipidemia. First, we found that plasma adiponectin levels decreased according to CDH13 genotypes, in order to from major AA to minor TT types. Although the mechanism of the transcriptional regulation of CDH13 is not clear, a nucleotide variant in the promoter region is associated with increased promoter activity, and also plays a crucial role in its expression.18,19 Even though rs3865188 is not located in the promoter region of CDH13, we demonstrated in the present study that this variant is positively associated with adiponectin levels.18 This is similar to the findings of previous studies, and our results suggest that the CDH13 variant rs3865188 may regulate adiponectin levels. The traditional cardiovascular risk factors, including age, sex, hypertension, smoking, hypercholesterolemia, and body mass index, have been reported.20 Another study investigated the role of T-cadherin expression in regulating adiponectin levels, and the involvement of CDH13 or adiponectin levels in the development of cardiometabolic diseases.11 Furthermore, several reports have found that CDH13 variants have modulating effects on metabolic traits, and they increase the risk of metabolic syndrome, diabetes mellitus, and ischemic stroke. In contrast to these previous studies, rs3865188 variants were not associated with risk of cardiometabolic diseases including dyslipidemia in our study. However, TG, TG/HDL, and adiponectin levels according to CDH13 genotypes or allele types might be changed by statin administration. This suggests that increases in plasma adiponectin levels upon statin administration are strongly associated with particular genes.
We also found that CDH13 SNP (rs3865188) is strongly associated with metabolic traits, including BP and plasma levels of FBS, insulin, and TG and that adiponectin levels are not negatively associated with dyslipidemia described by TG/HDL. Recently, an association between a CDH13 SNP and BP was identified in a subsequent genome-wide association study. In particular, carriers of the minor allele of CDH13 rs11646213 showed a decreased risk of hypertension, while carriers of rs3096277 and rs254340 (located in intron 11) showed an association with BP.21 Evidence of the involvement of CDH13 in BP regulation was demonstrated in another population-based cohort (Framingham Heart Study).21 In hypertension subjects, minor allele carriers of rs12444338 had a lower risk of hypertension, although the association was marginal after adjusting for confounders. In addition, the mean carotid intima-media thickness (IMT) was significantly associated with rs12444338 (p=0.02) and rs1048612 (p=0.02).22 In this study, genotypes of the SNP rs3865188 were also associated with DBP. Interestingly, we observed lower insulin levels with increased adiponectin levels in statin-treated subjects with the T allele, compared to statin-free groups with the A allele. CDH13 strongly influences circulating adiponectin levels and is associated with a beneficial metabolic profile: the positive relationship between adiponectin and insulin sensitivity in East Asian populations appears to be causal.23 In the present study, plasma adiponectin levels were negatively associated with TG and TG/HDL ratio in patients with the AT genotype in the statin-free group. In the statin group, however, the effect of the AT genotype disappeared, because adiponectin levels was increased by statin administration. We found that statin treatment significantly decreased TC, LDLc, TG/HDL ratio, and HDL3b fraction, compared to statin-free individuals, and increased adiponectin in persons having mutant alleles of the CDH13 gene. In general, a shifting toward small-sized HDL was changed by serum TG or TC elevation. Besides, serum TG level is a more important factor to change the components of small HDL such as HDL3b or HDL3c, compared to serum TC level.16 Even though we do not know whether adiponectin causes these lipid abnormalities and, thus, whether it is partly responsible for atherogenic risk, high TG/HDL is associated with low plasma adiponectin concentrations in nondiabetic women.24 In our study, the interaction between CDH13 gene and statin may effect HDL subpopulations according to changing plasma TG and TC levels.
Many studies have revealed that statin administered before or after CAD or stroke onset can decrease mortality and enhance the short-term and/or long-term outcomes of ischemic stroke.25,26 However, inflammation is not included in the list of classic risk factors for atherosclerosis, although it seems to be an additional important therapeutic target for the reduction of cardiovascular risk. The secretion of biologically active molecules including MCP-1 has an ominous impact on atherogenesis. Nevertheless, adiponectin has a protective effect against the development of cardiovascular disease, and its levels are reduced in metabolic syndrome.27,28 The use of drugs such as statin for treating dyslipidemia and its modifying effects on lipid profiles has been the focus of recent studies, which suggest that therapeutic targeting of inflammation may be used as a strategy to decrease cardiovascular risk.29 Some reports show that the use of rosuvastatin, a HMG-CoA reductase inhibitor, leads to a significant reduction in cardiovascular events in individuals without dyslipidemia with elevated high-sensitivity C-reactive protein levels, a marker of systemic inflammation.30 In the present study, statin did not affect inflammatory cytokines, such as MCP-1, leptin, visfatin, IL-6, and TNF-α. Meanwhile, patients in the statin group with the TT genotype exhibited higher MCP-1 levels than those in the statin-free group. Since plasma adiponectin level was increased by statin in TT genotypes, we assumed that adiponectin and MCP-1 was balanced to offset the increased CVD.
The impact of genetic polymorphisms on statin treatment efficacy has been analyzed in several clinical trials, and the variants in more than 30 different genes have been investigated.15 Even though knowledge obtained in this field is quickly growing, results have not been replicated in larger patient groups.15 Only the APOE4 allele has been shown to be associated with a poor response to statin treatment. Other genes examined include only APOA5 or cholesterol 7-α hydroxylase. One report detected a potential variant within the APOE/C1/C3 gene cluster that could influence statin treatment efficacy, and another pilot study suggested that the rs4149056 variant within the SLCO1B1 gene had possible sex-dependent effects on statin treatment efficacy.31 CDH13 is highly expressed in several tissues, including the heart, aortic wall, neurons of the brain cortex and spinal cord, and small blood vessels, as well as in a variety of cell types, such as vascular endothelial cells, smooth muscle cells, pericytes, cardiomyocytes, and cancer cells.11,32 Reports on cellular signaling have observed that both LDL and adiponectin are specific ligands for T-cadherin, a product of CDH13. The binding of LDL or adiponectin to T-cadherin stimulates the nuclear factor-κB signaling pathway, which plays an important role in inflammation, and serves as a link between obesity and vascular disease.19,33 In addition, T-cadherin might modulate vascular remodeling, neointima formation, inflammation-related phenomena, and atherosclerosis development by regulating the adiponectin levels in the blood and various tissues.21 In the present study, we showed the contribution of variants of CDH13 to statin treatment efficacy. However, the genetic effects of CDH13 rs3865188 and the complexity of the development of cardiometabolic diseases was not evaluated in the present study, because there was not enough data to demonstrate an association between one variant of CDH13 and statin treatment.
There are several limitations of this study. First, we could not find any reports that provided an explanation of the association between the CDH13 polymorphism and statin treatment efficacy. There is abundant research demonstrating the association of adiponectin levels and the CDH13 gene in several populations. However, we observed that studies in which genes that affect statin treatment efficacy have been identified, including the APOE/C1/C3 gene cluster, do not provide sufficient data to demonstrate the association of CDH13 and statin treatment. A similar problem was encountered in our study. Second, only one variant of CDH13 (rs3865188) was examined in our study; this is not sufficient to effectively evaluate the association between the CDH13 gene and adiponectin levels. Third, we did not perform any analysis to predict the effects of pharmacotherapy, and the individual efficacy of a drug was determined through genetic analysis only.
In the present study, we found that the genotypes of CDH13 play a crucial role in regulating adiponectin levels with plasma lipid profiles undergoing statin treatment in Korean CVD and dyslipidemia patients. The results of genetic analyses conducted in the future may help clinicians select the most effective and safe treatment for each individual patient. Although the economical and health benefits of such approaches are evident, we are still at the early stages of this research and only beginning to understand the genetic factors that determine statin treatment efficacy. Nevertheless, the novel variations within CDH13 may be utilized for better clinical management of coronary artery diseases in the future.
ACKNOWLEDGEMENTS
This study was funded by the Ministry of Health and Welfare (A000385) and Mid-career Researcher Program through NRF grant funded by the MEST (2010-0000147), Republic of Korea.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Orlik B, Madej P, Owczarek A, Skałba P, Chudek J, Olszanecka-Glinianowicz M. Plasma omentin and adiponectin levels as markers of adipose tissue dysfunction in normal weight and obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2014;81:529–535. doi: 10.1111/cen.12381. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura Y, Ueshima H, Okuda N, Miura K, Kita Y, Okamura T, et al. Relation of Serum Leptin and Adiponectin Level to Serum C-Reactive Protein: The INTERLIPID Study. Int J Vasc Med. 2013;2013:601364. doi: 10.1155/2013/601364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morisaki H, Yamanaka I, Iwai N, Miyamoto Y, Kokubo Y, Okamura T, et al. CDH13 gene coding T-cadherin influences variations in plasma adiponectin levels in the Japanese population. Hum Mutat. 2012;33:402–410. doi: 10.1002/humu.21652. [DOI] [PubMed] [Google Scholar]
- 4.Kasahara DI, Williams AS, Benedito LA, Ranscht B, Kobzik L, Hug C, et al. Role of the adiponectin binding protein, T-cadherin (cdh13), in pulmonary responses to subacute ozone. PLoS One. 2013;8:e65829. doi: 10.1371/journal.pone.0065829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uetani E, Tabara Y, Kawamoto R, Onuma H, Kohara K, Osawa H, et al. CDH13 genotype-dependent association of high-molecular weight adiponectin with all-cause mortality: the J-SHIPP study. Diabetes Care. 2014;37:396–401. doi: 10.2337/dc13-1658. [DOI] [PubMed] [Google Scholar]
- 6.Mavroconstanti T, Johansson S, Winge I, Knappskog PM, Haavik J. Functional properties of rare missense variants of human CDH13 found in adult attention deficit/hyperactivity disorder (ADHD) patients. PLoS One. 2013;8:e71445. doi: 10.1371/journal.pone.0071445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fredette BJ, Miller J, Ranscht B. Inhibition of motor axon growth by T-cadherin substrata. Development. 1996;122:3163–3171. doi: 10.1242/dev.122.10.3163. [DOI] [PubMed] [Google Scholar]
- 8.Rubina K, Kalinina N, Potekhina A, Efimenko A, Semina E, Poliakov A, et al. T-cadherin suppresses angiogenesis in vivo by inhibiting migration of endothelial cells. Angiogenesis. 2007;10:183–195. doi: 10.1007/s10456-007-9072-2. [DOI] [PubMed] [Google Scholar]
- 9.Kyriakakis E, Philippova M, Joshi MB, Pfaff D, Bochkov V, Afonyushkin T, et al. T-cadherin attenuates the PERK branch of the unfolded protein response and protects vascular endothelial cells from endoplasmic reticulum stress-induced apoptosis. Cell Signal. 2010;22:1308–1316. doi: 10.1016/j.cellsig.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Philippova M, Ivanov D, Joshi MB, Kyriakakis E, Rupp K, Afonyushkin T, et al. Identification of proteins associating with glycosylphosphatidylinositol-anchored T-cadherin on the surface of vascular endothelial cells: role for Grp78/BiP in T-cadherin-dependent cell survival. Mol Cell Biol. 2008;28:4004–4017. doi: 10.1128/MCB.00157-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung CM, Lin TH, Chen JW, Leu HB, Yang HC, Ho HY, et al. A genome-wide association study reveals a quantitative trait locus of adiponectin on CDH13 that predicts cardiometabolic outcomes. Diabetes. 2011;60:2417–2423. doi: 10.2337/db10-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He GX, Tan W. High-dose atorvastatin pretreatment could diminishes microvascular impairment in patients undergoing elective percutaneous coronary intervention. J Geriatr Cardiol. 2013;10:355–360. doi: 10.3969/j.issn.1671-5411.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nusca A, Melfi R, Patti G, Sciascio GD. Statin loading before percutaneous coronary intervention: proposed mechanisms and applications. Future Cardiol. 2010;6:579–589. doi: 10.2217/fca.10.77. [DOI] [PubMed] [Google Scholar]
- 14.Chung SD, Tsai MC, Lin HC, Kang JH. Statin use and clinical outcomes among pneumonia patients. Clin Microbiol Infect. 2014;20:879–885. doi: 10.1111/1469-0691.12544. [DOI] [PubMed] [Google Scholar]
- 15.Vrablík M, Hubáček JA, Dlouhá D, Lánská V, Rynekrová J, Zlatohlávek L, et al. Impact of variants within seven candidate genes on statin treatment efficacy. Physiol Res. 2012;61:609–617. doi: 10.33549/physiolres.932341. [DOI] [PubMed] [Google Scholar]
- 16.Lee M, Jang Y, Kim K, Cho H, Jee SH, Park Y, et al. Relationship between HDL3 subclasses and waist circumferences on the prevalence of metabolic syndrome: KMSRI-Seoul Study. Atherosclerosis. 2010;213:288–293. doi: 10.1016/j.atherosclerosis.2010.07.056. [DOI] [PubMed] [Google Scholar]
- 17.Sull JW, Kim HJ, Yun JE, Kim G, Park EJ, Kim S, et al. Serum adiponectin is associated with family history of diabetes independently of obesity and insulin resistance in healthy Korean men and women. Eur J Endocrinol. 2009;160:39–43. doi: 10.1530/EJE-08-0603. [DOI] [PubMed] [Google Scholar]
- 18.Jee SH, Sull JW, Lee JE, Shin C, Park J, Kimm H, et al. Adiponectin concentrations: a genome-wide association study. Am J Hum Genet. 2010;87:545–552. doi: 10.1016/j.ajhg.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su TC, Chien KL, Jeng JS, Chen MF, Hsu HC, Torng PL, et al. Age- and gender-associated determinants of carotid intima-media thickness: a community-based study. J Atheroscler Thromb. 2012;19:872–880. doi: 10.5551/jat.10728. [DOI] [PubMed] [Google Scholar]
- 21.Org E, Eyheramendy S, Juhanson P, Gieger C, Lichtner P, Klopp N, et al. Genome-wide scan identifies CDH13 as a novel susceptibility locus contributing to blood pressure determination in two European populations. Hum Mol Genet. 2009;18:2288–2296. doi: 10.1093/hmg/ddp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JH, Shin DJ, Park S, Kang SM, Jang Y, Lee SH. Association between CDH13 variants and cardiometabolic and vascular phenotypes in a Korean population. Yonsei Med J. 2013;54:1305–1312. doi: 10.3349/ymj.2013.54.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao H, Kim YM, Chen P, Igase M, Kawamoto R, Kim MK, et al. Genetic variation in CDH13 is associated with lower plasma adiponectin levels but greater adiponectin sensitivity in East Asian populations. Diabetes. 2013;62:4277–4283. doi: 10.2337/db13-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsubara M, Maruoka S, Katayose S. Decreased plasma adiponectin concentrations in women with dyslipidemia. J Clin Endocrinol Metab. 2002;87:2764–2769. doi: 10.1210/jcem.87.6.8550. [DOI] [PubMed] [Google Scholar]
- 25.Song B, Wang Y, Zhao X, Liu L, Wang C, Wang A, et al. Association between statin use and short-term outcome based on severity of ischemic stroke: a cohort study. PLoS One. 2014;9:e84389. doi: 10.1371/journal.pone.0084389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Q, Ruan ZR, Jiang B, Yuan H, Zeng S. Simvastatin pharmacokinetics in healthy Chinese subjects and its relations with CYP2C9, CYP3A5, ABCB1, ABCG2 and SLCO1B1 polymorphisms. Pharmazie. 2013;68:124–128. [PubMed] [Google Scholar]
- 27.Lobo SM, Quinto BM, Oyama L, Nakamichi R, Ribeiro AB, Zanella MT, et al. TNF-α modulates statin effects on secretion and expression of MCP-1, PAI-1 and adiponectin in 3T3-L1 differentiated adipocytes. Cytokine. 2012;60:150–156. doi: 10.1016/j.cyto.2012.04.039. [DOI] [PubMed] [Google Scholar]
- 28.Aiello RJ, Bourassa PA, Lindsey S, Weng W, Natoli E, Rollins BJ, et al. Monocyte chemoattractant protein-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:1518–1525. doi: 10.1161/01.atv.19.6.1518. [DOI] [PubMed] [Google Scholar]
- 29.Ridker PM, Morrow DA. C-reactive protein, inflammation, and coronary risk. Cardiol Clin. 2003;21:315–325. doi: 10.1016/s0733-8651(03)00079-1. [DOI] [PubMed] [Google Scholar]
- 30.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 31.Hubacek JA, Dlouha D, Adámkova V, Lanska V, Ceska R, Vrablik M. Possible gene-gender interaction between the SLCO1B1 polymorphism and statin treatment efficacy. Neuro Endocrinol Lett. 2012;33(Suppl 2):22–25. [PubMed] [Google Scholar]
- 32.Ivanov D, Philippova M, Antropova J, Gubaeva F, Iljinskaya O, Tararak E, et al. Expression of cell adhesion molecule T-cadherin in the human vasculature. Histochem Cell Biol. 2001;115:231–242. doi: 10.1007/s004180100252. [DOI] [PubMed] [Google Scholar]
- 33.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]