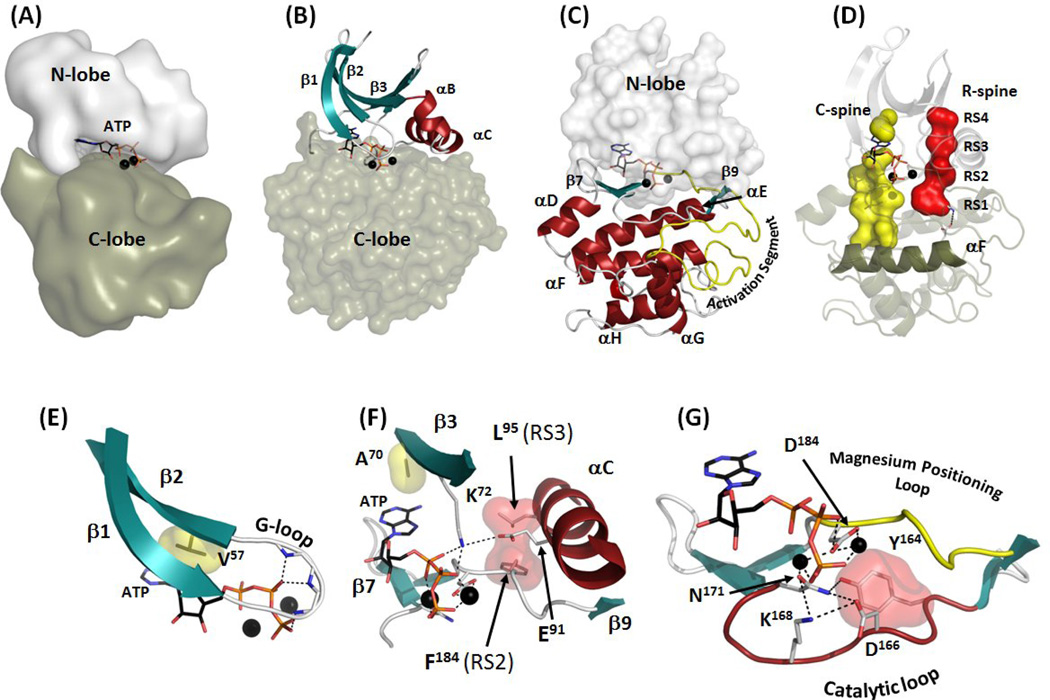
Figure 1.
Internal architecture of the protein kinase core. (A) Bilobal structure of protein kinase core shows ATP imbedded in the deep cleft between the smaller N-lobe (white surface) and the larger C-lobe (olive surface). (B) The N-lobe has five β-strands (teal) and a large α-C helix (red). (C) The C-lobe is predominantly helical with a large Activation Segment between β8 and αF (yellow). (D) Two “hydrophobic spines” are assembled in active kinases: The R-spine (red surface) that contains four residues (RS1 through RS4) is bound to the αF-helix via conserved hydrogen bond and is correctly assembled only in the active kinase. The C-spine (yellow surface) is completed by adenine ring of ATP. The spines connect the two lobes and span across the molecule from the large αF-helix in the C-lobe to the rigid β-sheet in the N-lobe. (E) Glycine-rich Loop (G-loop) lies between β1 and β2 and coordinates the phosphate groups of ATP. β2-strand also contains the conserved Val57 that is a part of the C-spine. (F) β3-strand contains two universally conserved residues, Ala70 and Lys72. The former is a part of the C-spine and the latter coordinates the α- and β-phosphates of ATP and binds to the invariant Glu91 from the αC-helix, stabilizing the R-spine (red surface) and ordering the αC-helix for catalysis. (G) Residues that are directly involved in the phosphotransfer are in the Catalytic loop between β6 and β9 (red) with Asp184 coming from the Magnesium Positioning Loop (yellow).