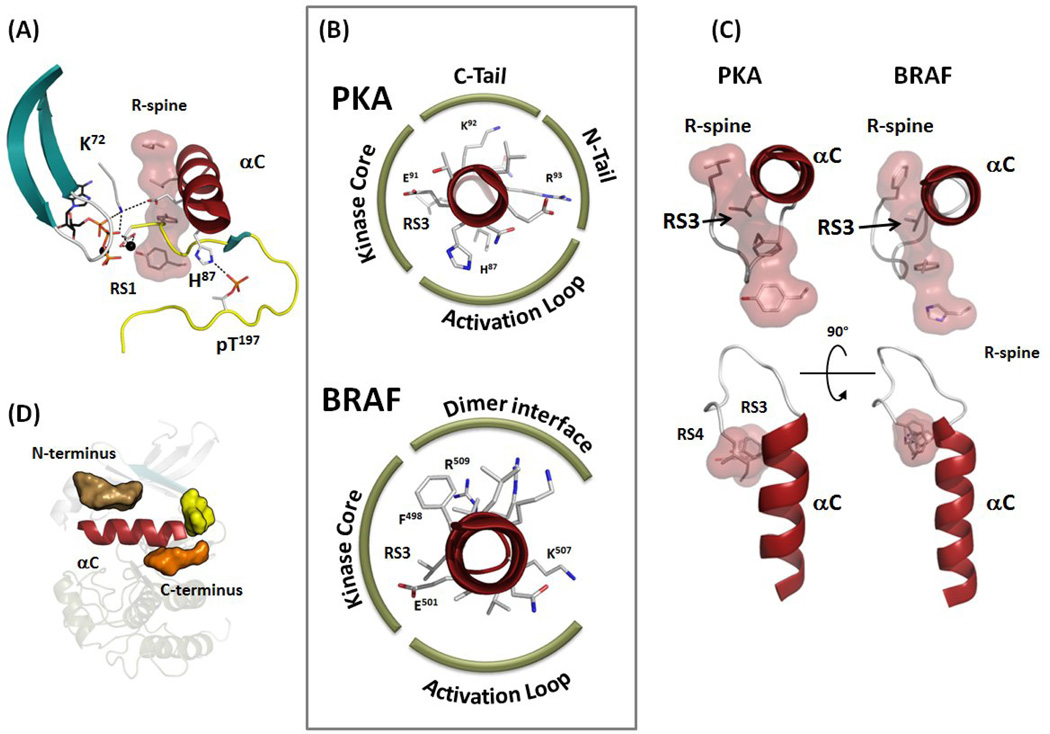
Figure 3.
Multiple ways to stabilize the αC-helix. (A) N-terminal part of the αC-helix is often bound to the phosphorylated residue in the Activation Loop, while the middle part of the helix is stabilized by the conserved Lys72-Glu91 salt bridge. (B) αC-helices of PKA and BRAF shown as a wheel highlighting their interactions. While interactions of the αC-helix with the kinase core is usually conserved, other surfaces of the helix have different binding partners. (C) C terminus of the αC-helix in all kinases is anchored to the kinase core by the αC-β4 Loop and via integration of its RS3 residue with the R-spine. (D) Conserved pockets flank the surface of the αC-helix as detected by Thompson et al. [46]. One pocket is located at the N terminus (tan surface) and two pockets at the C terminus (yellow and orange surfaces).