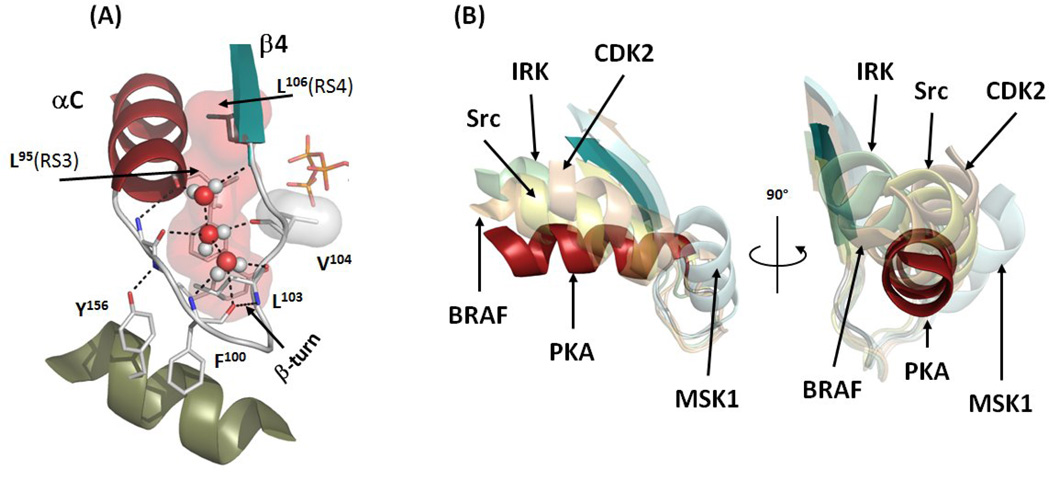
Figure 4.
αC-β4 Loop is a pivot point for the αC-helix movement. (A) The αC-β4 Loop (white) connects aC-helix (red) and β4 (teal). It is anchored to the αE-helix (olive) via interaction with conserved Tyr156. The geometry of the loop is highly conserved with the β-turn between Phe100 and Leu103 and three water molecules (red and white spheres). The assembled R-spine is shown as a red surface. Val104 is shown as a white surface. (B) The αC-β4 Loops of five inactive kinases (transparent cartoons) in comparison with the αC-β4 Loop of active PKA (red and teal). The structures were aligned by their C-lobes. The following structures were used: PKA (PDBID:1ATP), BRAF (PDBID:1UWH), Src (PDBID:2SRC), IRK (PDBID:1IRK), CDK2 (PDBID:1B39), MSK1 (PDBID:1VZO).