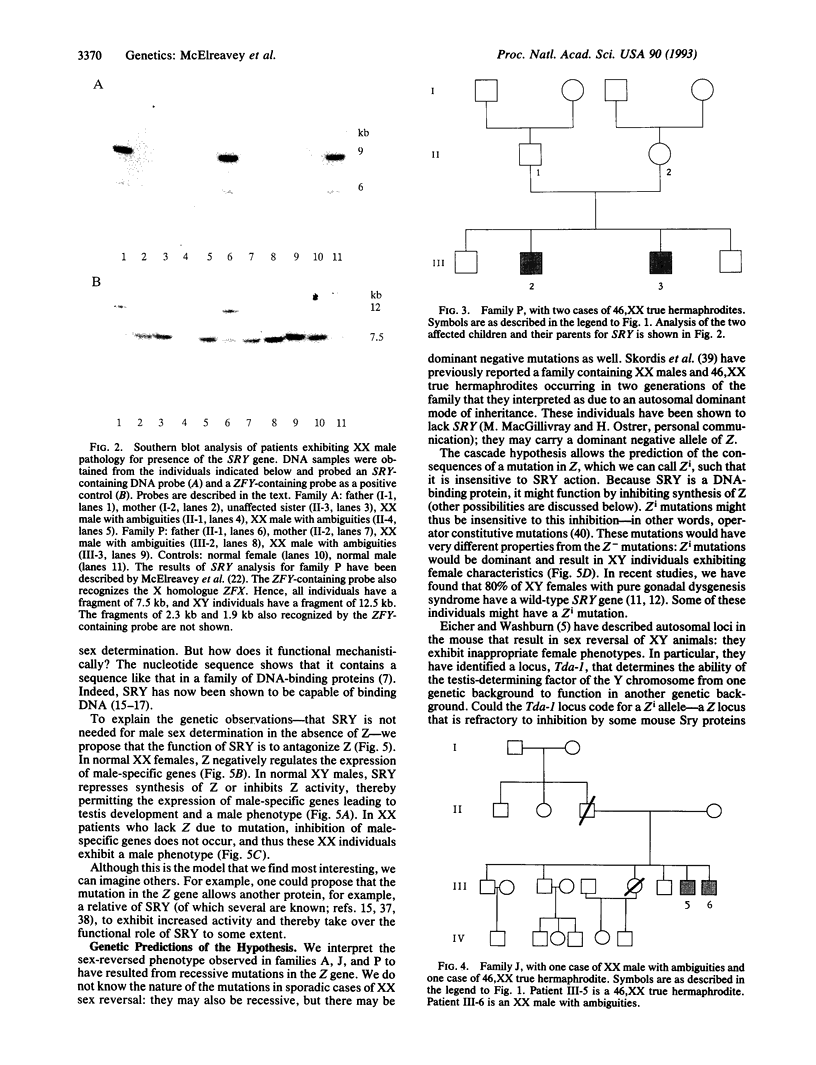
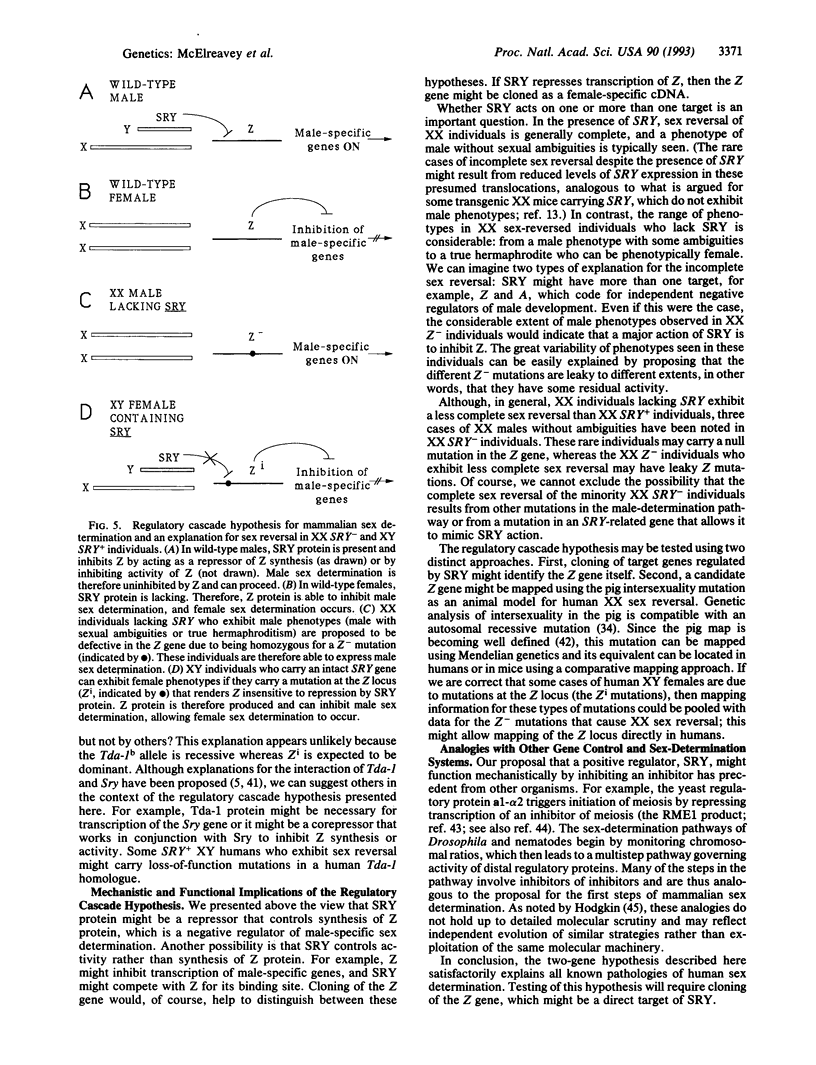
Abstract
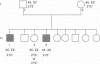
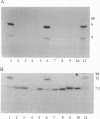
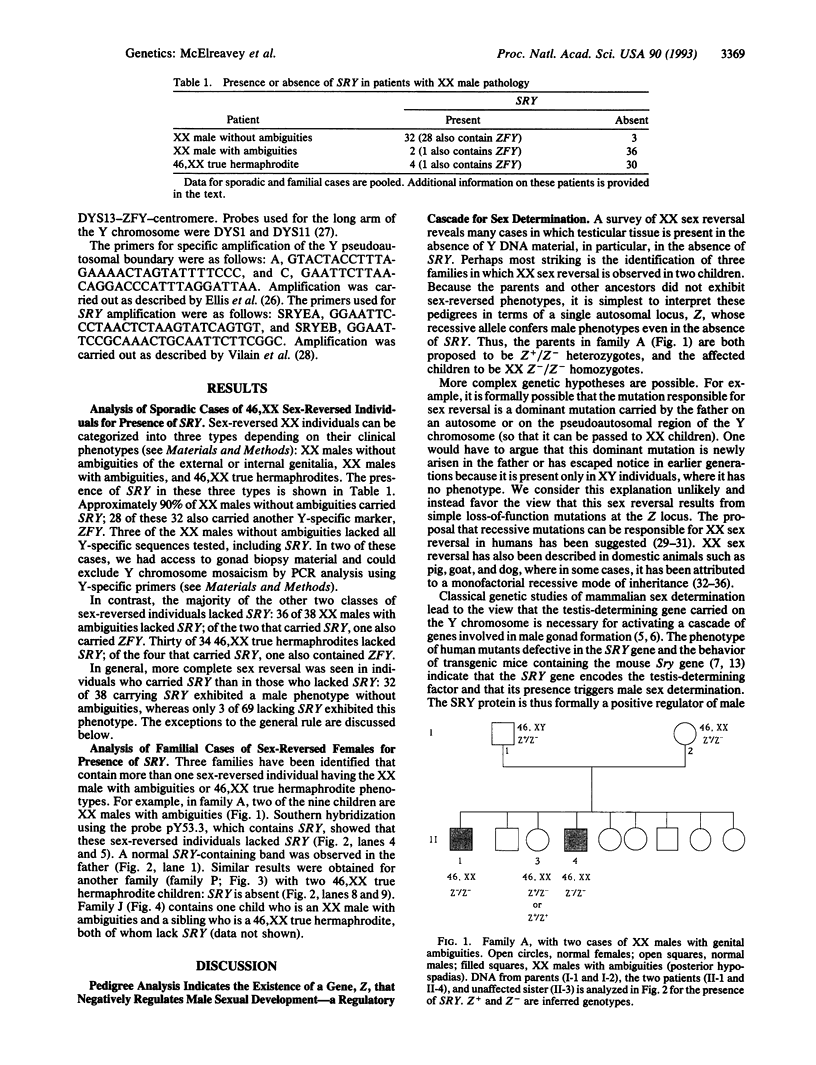
The mammalian Y chromosome carries the SRY gene, which determines testis formation. Here we review data on individuals who are XX but exhibit male characteristics: some have SRY; others do not. We have analyzed three families containing more than one such individual and show that these individuals lack SRY. Pedigree analysis leads to the hypothesis that they carry recessive mutations (in a gene termed Z) that allow expression of male characteristics. We propose that wild-type Z product is a negative regulator of male sex determination and is functional in wild-type females. In males, SRY product represses or otherwise negatively regulates Z and thereby allows male sex determination. This hypothesis can also explain other types of sex reversal in mammals, in particular, XY females containing SRY. Some of these individuals may have mutations at the Z locus rendering them insensitive to SRY. Recessive mutations (such as the polled mutation of goats) leading to sex reversal are known in a variety of animals and might be used to map and ultimately clone the human Z gene.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbas N. E., Toublanc J. E., Boucekkine C., Toublanc M., Affara N. A., Job J. C., Fellous M. A possible common origin of "Y-negative" human XX males and XX true hermaphrodites. Hum Genet. 1990 Mar;84(4):356–360. doi: 10.1007/BF00196234. [DOI] [PubMed] [Google Scholar]
- Affara N. A., Florentin L., Morrison N., Kwok K., Mitchell M., Cook A., Jamieson D., Glasgow L., Meredith L., Boyd E. Regional assignment of Y-linked DNA probes by deletion mapping and their homology with X-chromosome and autosomal sequences. Nucleic Acids Res. 1986 Jul 11;14(13):5353–5373. doi: 10.1093/nar/14.13.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armendares S., Salamanca F., Cantú J. M., Del Castillo V., Nava S., Dominguez-de-la-Piedra E., Cortés-Gallegos V., Gallegos A., Cervantes C., Parra A. Familial true hermaphrodism in three siblings: clinical, cytogenetic, histological and hormonal studies. Humangenetik. 1975 Sep 10;29(2):99–109. doi: 10.1007/BF00430346. [DOI] [PubMed] [Google Scholar]
- Banuett F. Identification of genes governing filamentous growth and tumor induction by the plant pathogen Ustilago maydis. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3922–3926. doi: 10.1073/pnas.88.9.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berta P., Hawkins J. R., Sinclair A. H., Taylor A., Griffiths B. L., Goodfellow P. N., Fellous M. Genetic evidence equating SRY and the testis-determining factor. Nature. 1990 Nov 29;348(6300):448–450. doi: 10.1038/348448A0. [DOI] [PubMed] [Google Scholar]
- Burgoyne P. S. Role of mammalian Y chromosome in sex determination. Philos Trans R Soc Lond B Biol Sci. 1988 Dec 1;322(1208):63–72. doi: 10.1098/rstb.1988.0114. [DOI] [PubMed] [Google Scholar]
- Castrop J., van Norren K., Clevers H. A gene family of HMG-box transcription factors with homology to TCF-1. Nucleic Acids Res. 1992 Feb 11;20(3):611–611. doi: 10.1093/nar/20.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribiu E. P., Chaffaux S. L'intersexualité chez les mammifères domestiques. Reprod Nutr Dev. 1990;Suppl 1:51s–61s. [PubMed] [Google Scholar]
- Eicher E. M., Washburn L. L. Genetic control of primary sex determination in mice. Annu Rev Genet. 1986;20:327–360. doi: 10.1146/annurev.ge.20.120186.001551. [DOI] [PubMed] [Google Scholar]
- Ellis N. A., Goodfellow P. J., Pym B., Smith M., Palmer M., Frischauf A. M., Goodfellow P. N. The pseudoautosomal boundary in man is defined by an Alu repeat sequence inserted on the Y chromosome. Nature. 1989 Jan 5;337(6202):81–84. doi: 10.1038/337081a0. [DOI] [PubMed] [Google Scholar]
- Giese K., Cox J., Grosschedl R. The HMG domain of lymphoid enhancer factor 1 bends DNA and facilitates assembly of functional nucleoprotein structures. Cell. 1992 Apr 3;69(1):185–195. doi: 10.1016/0092-8674(92)90129-z. [DOI] [PubMed] [Google Scholar]
- Goodfellow P. N., Darling S. M. Genetics of sex determination in man and mouse. Development. 1988 Feb;102(2):251–258. doi: 10.1242/dev.102.2.251. [DOI] [PubMed] [Google Scholar]
- Guellaen G., Casanova M., Bishop C., Geldwerth D., Andre G., Fellous M., Weissenbach J. Human XX males with Y single-copy DNA fragments. Nature. 1984 Jan 12;307(5947):172–173. doi: 10.1038/307172a0. [DOI] [PubMed] [Google Scholar]
- Hamerton J. L., Dickson J. M., Pollard C. E., Grieves S. A., Short R. V. Genetic intersexuality in goats. J Reprod Fertil Suppl. 1969 May;7(Suppl):25–51. [PubMed] [Google Scholar]
- Harley V. R., Jackson D. I., Hextall P. J., Hawkins J. R., Berkovitz G. D., Sockanathan S., Lovell-Badge R., Goodfellow P. N. DNA binding activity of recombinant SRY from normal males and XY females. Science. 1992 Jan 24;255(5043):453–456. doi: 10.1126/science.1734522. [DOI] [PubMed] [Google Scholar]
- Hawkins J. R., Taylor A., Berta P., Levilliers J., Van der Auwera B., Goodfellow P. N. Mutational analysis of SRY: nonsense and missense mutations in XY sex reversal. Hum Genet. 1992 Feb;88(4):471–474. doi: 10.1007/BF00215684. [DOI] [PubMed] [Google Scholar]
- Hodgkin J. Sex determination compared in Drosophila and Caenorhabditis. Nature. 1990 Apr 19;344(6268):721–728. doi: 10.1038/344721a0. [DOI] [PubMed] [Google Scholar]
- Hodgkin J. Sex determination compared in Drosophila and Caenorhabditis. Nature. 1990 Apr 19;344(6268):721–728. doi: 10.1038/344721a0. [DOI] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Jäger R. J., Anvret M., Hall K., Scherer G. A human XY female with a frame shift mutation in the candidate testis-determining gene SRY. Nature. 1990 Nov 29;348(6300):452–454. doi: 10.1038/348452a0. [DOI] [PubMed] [Google Scholar]
- Koopman P., Gubbay J., Vivian N., Goodfellow P., Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991 May 9;351(6322):117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- McElreavey K. D., Vilain E., Boucekkine C., Vidaud M., Jaubert F., Richaud F., Fellous M. XY sex reversal associated with a nonsense mutation in SRY. Genomics. 1992 Jul;13(3):838–840. doi: 10.1016/0888-7543(92)90164-n. [DOI] [PubMed] [Google Scholar]
- McElreavey K., Rappaport R., Vilain E., Abbas N., Richaud F., Lortat-Jacob S., Berger R., Le Coniat M., Boucekkine C., Kucheria K. A minority of 46,XX true hermaphrodites are positive for the Y-DNA sequence including SRY. Hum Genet. 1992 Sep-Oct;90(1-2):121–125. doi: 10.1007/BF00210754. [DOI] [PubMed] [Google Scholar]
- McElreavy K., Vilain E., Abbas N., Costa J. M., Souleyreau N., Kucheria K., Boucekkine C., Thibaud E., Brauner R., Flamant F. XY sex reversal associated with a deletion 5' to the SRY "HMG box" in the testis-determining region. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):11016–11020. doi: 10.1073/pnas.89.22.11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A. P., Herskowitz I. Activation of meiosis and sporulation by repression of the RME1 product in yeast. 1986 Feb 27-Mar 5Nature. 319(6056):738–742. doi: 10.1038/319738a0. [DOI] [PubMed] [Google Scholar]
- Nasrin N., Buggs C., Kong X. F., Carnazza J., Goebl M., Alexander-Bridges M. DNA-binding properties of the product of the testis-determining gene and a related protein. Nature. 1991 Nov 28;354(6351):317–320. doi: 10.1038/354317a0. [DOI] [PubMed] [Google Scholar]
- Page D. C., Mosher R., Simpson E. M., Fisher E. M., Mardon G., Pollack J., McGillivray B., de la Chapelle A., Brown L. G. The sex-determining region of the human Y chromosome encodes a finger protein. Cell. 1987 Dec 24;51(6):1091–1104. doi: 10.1016/0092-8674(87)90595-2. [DOI] [PubMed] [Google Scholar]
- Palmer M. S., Sinclair A. H., Berta P., Ellis N. A., Goodfellow P. N., Abbas N. E., Fellous M. Genetic evidence that ZFY is not the testis-determining factor. Nature. 1989 Dec 21;342(6252):937–939. doi: 10.1038/342937a0. [DOI] [PubMed] [Google Scholar]
- Seboun E., Leroy P., Casanova M., Magenis E., Boucekkine C., Disteche C., Bishop C., Fellous M. A molecular approach to the study of the human Y chromosome and anomalies of sex determination in man. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):237–248. doi: 10.1101/sqb.1986.051.01.029. [DOI] [PubMed] [Google Scholar]
- Selden J. R., Wachtel S. S., Koo G. C., Haskins M. E., Patterson D. F. Genetic basis of XX male syndrome and XX true hermaphroditism: evidence in the dog. Science. 1978 Aug 18;201(4356):644–646. doi: 10.1126/science.675252. [DOI] [PubMed] [Google Scholar]
- Sex determination in mouse and man. Philos Trans R Soc Lond B Biol Sci. 1988 Dec 1;322(1208):1–157. [PubMed] [Google Scholar]
- Sinclair A. H., Berta P., Palmer M. S., Hawkins J. R., Griffiths B. L., Smith M. J., Foster J. W., Frischauf A. M., Lovell-Badge R., Goodfellow P. N. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990 Jul 19;346(6281):240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- Sittmann K., Breeuwsma A. J., te Brake J. H. On the inheritance of intersexuality in swine. Can J Genet Cytol. 1980;22(4):507–527. doi: 10.1139/g80-058. [DOI] [PubMed] [Google Scholar]
- Skordis N. A., Stetka D. G., MacGillivray M. H., Greenfield S. P. Familial 46,XX males coexisting with familial 46,XX true hermaphrodites in same pedigree. J Pediatr. 1987 Feb;110(2):244–248. doi: 10.1016/s0022-3476(87)80162-2. [DOI] [PubMed] [Google Scholar]
- Travis A., Amsterdam A., Belanger C., Grosschedl R. LEF-1, a gene encoding a lymphoid-specific protein with an HMG domain, regulates T-cell receptor alpha enhancer function [corrected]. Genes Dev. 1991 May;5(5):880–894. doi: 10.1101/gad.5.5.880. [DOI] [PubMed] [Google Scholar]
- Vergnaud G., Page D. C., Simmler M. C., Brown L., Rouyer F., Noel B., Botstein D., de la Chapelle A., Weissenbach J. A deletion map of the human Y chromosome based on DNA hybridization. Am J Hum Genet. 1986 Feb;38(2):109–124. [PMC free article] [PubMed] [Google Scholar]
- Vilain E., McElreavey K., Jaubert F., Raymond J. P., Richaud F., Fellous M. Familial case with sequence variant in the testis-determining region associated with two sex phenotypes. Am J Hum Genet. 1992 May;50(5):1008–1011. [PMC free article] [PubMed] [Google Scholar]
- Wachtel S. S., Basrur P., Koo G. C. Recessive male-determining genes. Cell. 1978 Sep;15(1):279–281. doi: 10.1016/0092-8674(78)90103-4. [DOI] [PubMed] [Google Scholar]
- Washburn L. L., Eicher E. M. Normal testis determination in the mouse depends on genetic interaction of a locus on chromosome 17 and the Y chromosome. Genetics. 1989 Sep;123(1):173–179. doi: 10.1093/genetics/123.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Chapelle A. The Y-chromosomal and autosomal testis-determining genes. Development. 1987;101 (Suppl):33–38. [PubMed] [Google Scholar]
- de la Chapelle A. The etiology of maleness in XX men. Hum Genet. 1981;58(1):105–116. doi: 10.1007/BF00284157. [DOI] [PubMed] [Google Scholar]
- de la Chapelle A., Tippett P. A., Wetterstrand G., Page D. Genetic evidence of X-Y interchange in a human XX male. Nature. 1984 Jan 12;307(5947):170–171. doi: 10.1038/307170a0. [DOI] [PubMed] [Google Scholar]