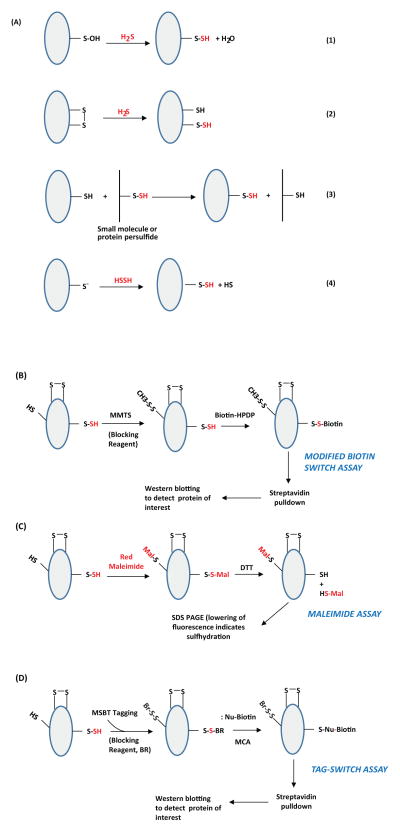
Figure 1. Potential mechanisms of protein sulfhydration and its detection.(A) Mechanisms for sulfydration/persulfidation.
Sulfhydration can occur by the reaction of sulfide with oxidized cysteine residues such as cysteine sulfenic acid or disulfides (Reactions 1 and 2 respectively). Sulfhydration may also occur when an existing persulfide (on either a small molecule or a protein) reacts with a cysteine thiol (Reaction 3). Reaction of H2S2 with cysteine thiolates may also lead to sulfhydration (Reaction 4). (B) The modified biotin switch assay. The illustration depicts a protein with unmodified cysteines (-SH), sulfhydrated cysteines (-SSH) and disulfide bonded cysteines (S-S). Purified protein, or cell or tissue lysate, is incubated with methyl methanethiosulfonate (MMTS), to block unmodified cysteines. Unreacted MMTS is then removed by acetone precipitation or by gel filtration followed by treatment of the sulfhydrated protein with biotin-HPDP, which reacts with the protein at the site of sulfhydration. The biotinylated protein is enriched using streptavidin conjugates and analyzed by western blot analysis. (C) Maleimide assay. In this assay, the protein is first immunoprecipitated and treated with a fluorescent version of maleimide, which reacts with thiols under conditions that preserve the native conformation of the protein. After removing excess maleimide, the reaction mixture is treated with dithiothreitol (DTT), which reduces the disulfide bond resulting in the removal of the maleimide and a decrease in fluorescence that can be observed by SDS-PAGE. (D) The tag switch assay. The assay is a variation of the modified biotin switch assay. The reaction mixture is treated with the thiol blocking reagent (BR): methylsulfonyl benzothiazole (MSBT), followed by treatment with a methylcyanoacetate (MCA) derivative that comprises a nucleophilic component and a biotin moiety as a reporter. The biotinylated protein is then captured using streptavidin beads and analyzed by western blotting. The modifications caused by sulfide or its derivatives are shown in red.