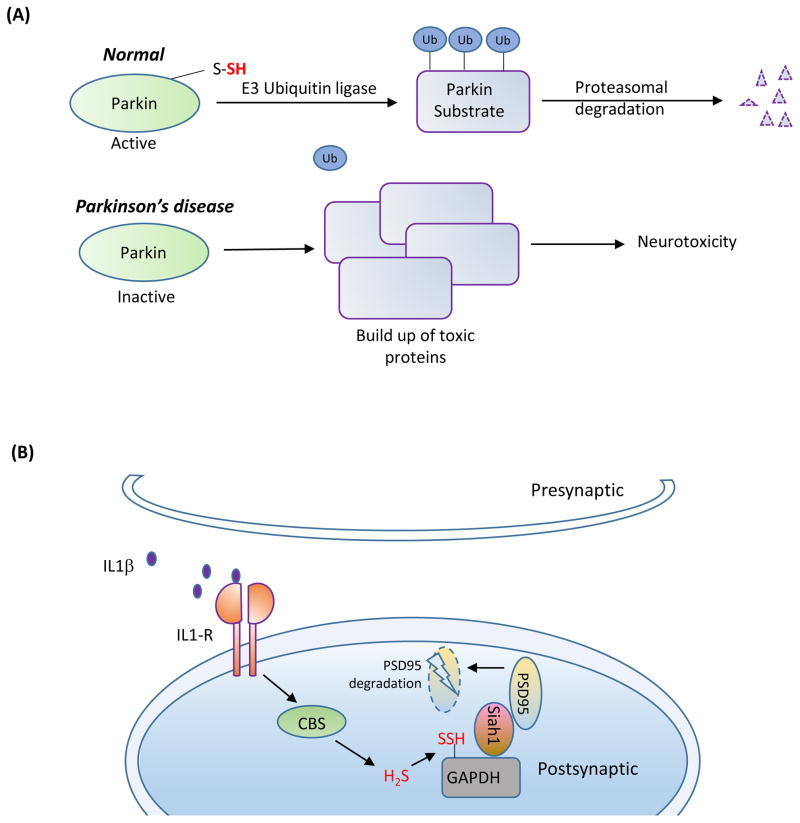
Figure 4. Sulfhydration in the brain.
(A) Sulfhydration is dysregulated in Parkinson’s disease (PD). In normal subjects, the E3 ubiquitin ligase, Parkin, is sulfhydrated under basal conditions, which enhances its catalytic activity. Parkin mediates ubiquitylation of substrates such as α-synuclein (a component of the Lewy bodies found in PD) and targets them for degradation. In sporadic forms of PD, sulfhydration of Parkin is diminished, leading to decreased catalytic activity, which results in accumulation of toxic proteins and neurotoxicity.(B) Sulfhydration regulates synaptic function. The proinflammtory cytokine interleukin-1β (IL-1β, purple circles) plays key roles in learning and memory and is involved in promoting long term potentiation (LTP). IL-1β activates the transcription factor specificity protein 1 (SP1), which stimulates the transcription of cystathionine β-synthase (CBS), the major H2S producing enzyme in the brain, leading to sulfhydration of the glycolytic enzyme glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Sulfhydrated GAPDH binds to seven in absentia homolog-1 (siah1), an E3 ubiquitin ligase, which targets post-synaptic density 95 protein (PSD95) for degradation. PSD95 is a scaffolding protein that participates in synaptic functions. Degradation of PSD95 leads to spine retraction and associated cognitive deficits.