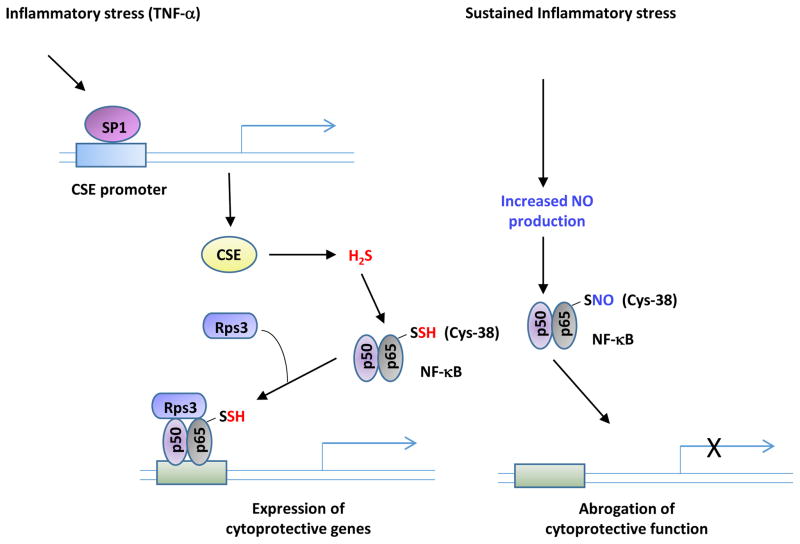
Figure 5. Reciprocity of sulfhydration and nitrosylation.
Sulfhydration and nitrosylation occur on reactive cysteines and as a result frequently modify the same residue. In general, sulfhydration and nitrosylation are functionally antagonistic although there are examples where both modifications elicit the same outcome. In several instances, sulfhydration precedes nitrosylation. During inflammatory conditions (left), cystathionine γ-lyase (CSE) expression is stimulated to produce hydrogen sulfide (H2S). H2S sulfhydrates the p65 subunit of the transcription factor NF-κB and promotes its association with its coactivator, the ribosomal protein S3 (rps3), to enhance expression of cytoprotective genes. If the inflammatory signals persist, the cells produce nitric oxide (NO), which nitrosylates p65 at the same residue and inhibits its DNA binding activity and cytoprotective functions.