FIGURE 2.
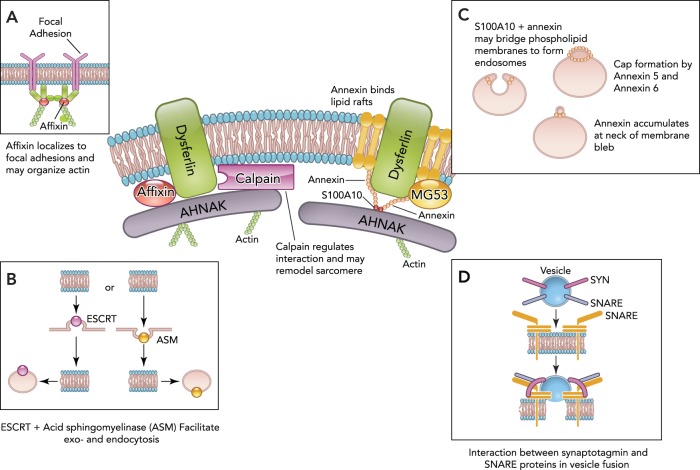
Major membrane repair proteins and their hypothesized roles in the repair process
Dysferlin's interaction with AHNAK is regulated by calpain, and cleavage of dysferlin can result in additional subunits that function in repair. Calpain may also regulate cytoskeletal structure and sarcomere remodeling. AHNAK may aid in cytoskeletal remodeling. MG53/TRIM72 and dysferlin form a vesicle lattice to close the wound. A: affixin, which also binds dysferlin, localizes to focal adhesions and may organize actin. B: ESCRT and acid sphingomyelinase (ASM) facilitate exocytosis and endocytosis. ESCRT has been found to be involved in both endocytosis and budding. ESCRT III can be recruited to the membrane, followed by blebbing of the membrane and shedding of the wound. ASM is secreted and cleaves sphingomyelin to generate ceramide, leading to membrane invagination of the injury site. C: the annexin and S100A10 complex binds dysferlin and may recruit AHNAK to the membrane due to annexin's ability to bind lipid rafts. Annexin/S100A10 may also bridge adjacent phospholipids to form endosomes. Annexin accumulates at the neck of membrane blebs to mediate microvesicle release. Annexin A6 may also “cap” the membrane repair patch. D: synaptotagmin and SNARE proteins interact at the plasma membrane via a conformational change in synaptotagmin present on synaptic vesicles to fuse the vesicles with the membrane.