Abstract
Parkinson's disease is a chronic, debilitating neurodegenerative movement disorder characterized by progressive degeneration of dopaminergic neurons in the substantia nigra pars compacta region in human midbrain. To date, oxidative stress is the well accepted concept in the etiology and progression of Parkinson's disease. Hence, the therapeutic agent is targeted against suppressing and alleviating the oxidative stress-induced cellular damage. Within the past decades, an explosion of research discoveries has reported on the protective mechanisms of flavonoids, which are plant-based polyphenols, in the treatment of neurodegenerative disease using both in vitro and in vivo models. In this paper, we have reviewed the literature on the neuroprotective mechanisms of flavonoids in protecting the dopaminergic neurons hence reducing the symptoms of this movement disorder. The mechanism reviewed includes effect of flavonoids in activation of endogenous antioxidant enzymes, suppressing the lipid peroxidation, inhibition of inflammatory mediators, flavonoids as a mitochondrial target therapy, and modulation of gene expression in neuronal cells.
1. Introduction
James Parkinson (1817), in his paper entitled “Essay on the Shaking Palsy,” described Parkinson's disease (PD) as a progressive neurodegenerative disease, characterized by selective loss of dopaminergic neurons in the human midbrain region known as the substantia nigra pars compacta (SNpc) [1]. Degeneration of dopaminergic neurons results in the depletion of the dopamine neurotransmitter production, which manifests clinically as motor dysfunctions such as tremors of hands, bradykinesia, postural instability, and rigidity [2]. Parkinson's disease is also associated with the presence of α-synuclein inclusions known as the Lewy bodies in the substantia nigra [3]. However, the basis of selective neuronal loss is still elusive since the disease is only diagnosed at the advanced stage.
The etiology of PD is not clearly defined as the disease does not present any clinical symptoms at the early stage [4]. In most instances, by the time the patient experiences the first clinical symptoms, about 50–70% of the dopaminergic neurons have been damaged or degenerated [5]. Up to now, the factors that trigger the onset of this disease still remain unknown [6]. Although, it has been proposed that PD may be caused by a genetic predisposition or environmental toxins, there is no direct evidence to substantiate these claims [6]. However, researchers have delineated the accumulation of abnormal or “toxic” protein in the neuronal cells to be one of the major causes of neuronal death [7]. There is also evidence to support the role of oxidative stress and imbalance in the natural antioxidant defense system, which could be contributing factors that support the formation and/or accumulation of abnormal or toxic proteins in the neurons [8]. Other factors associated with the pathogenesis of PD include living in rural areas [9], farming activity [10], and drinking well-water [11], as these factors can cause exposure to neurotoxic agents that are usually found in pesticides and environmental toxins [12]. On the contrary, some factors like regular consumption of caffeine and tea [13] as well as smoking [14] have been found to exert some protective effects against the onset of PD. A few prospective cohort studies as well as animal studies have suggested and proven that moderate to vigorous physical exercise can be a prophylaxis measure against PD [15, 16].
Flavonoids are water-soluble, broad polyphenol family found ubiquitously in plants, which contribute to the orange, blue, and purple color of fruits, flowers, and leaves [17]. Currently, more than 8000 flavonoid compounds have been identified and these are distributed in various kinds of food such as fruits, grains, nuts, green and black tea, and vegetables [18]. Primarily these flavonoids are synthesized by plants via the photosynthesis process and functions in protecting plants against reactive oxygen species (ROS) and are consumed by herbivores [19]. As shown in Table 1, flavonoids can be classified into six main subgroups: flavonones, flavones, isoflavones, flavanols, flavanones, and anthocyanidins [20].
Table 1.
| Subgroups | Types of flavonoids | Structures | Food sources |
|---|---|---|---|
| Flavone | Apigenin Luteolin |
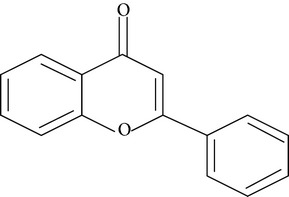
|
Apple skins Celery |
|
| |||
| Flavonol | Kaempferol Myricetin Quercetin Quercetin glycosides, rutin Quercetin glycosides, isoquercitrin |
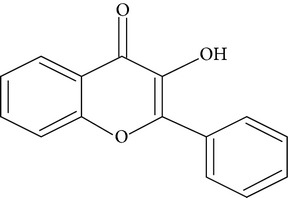
|
Broccoli Fruits peels Lettuce, olives, onions Buckwheat, citrus fruits Mango, apples, onion |
|
| |||
| Flavanol | (−)−Epicatechin (−)−Epicatechin 3-gallate (−)−Epigallocatechin (−)−Epigallocatechin 3-gallate (+)−Gallocatechin |
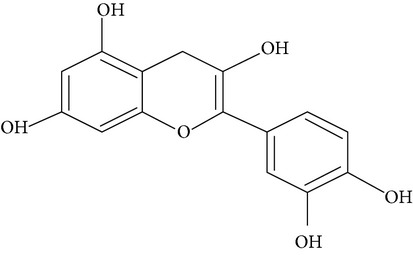
|
Berries, blueberries, fava beans, mature seeds, broccoli, Brussels sprouts |
|
| |||
| Flavanone | Hesperetin Fisetin Naringin Naringenin |
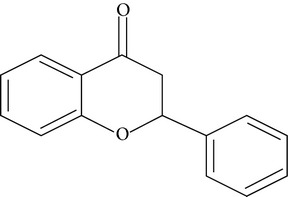
|
Citrus peel Citrus fruit |
|
| |||
| Anthocyanidin | Cyanidin Delphinidin Malvidin Pelargonidin Petunidin |
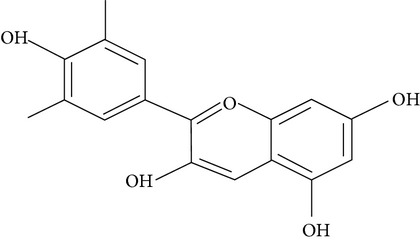
|
Berries Cherries Grapes Raspberries Red wines, strawberries, tea |
|
| |||
| Isoflavone | Daidzein Genistein Glycitein |
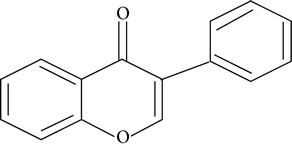
|
Soy bean |
Within the last 20 years, there has been an explosion of facts on the protective effects of flavonoids. However, the initial information on the health benefits of flavonoids relates to the profound antioxidant properties of these compounds [21]. To date, there is growing evidence that flavonoids do not only exhibit antioxidant effects, but also exhibit a variety of other protective effects such as antiapoptotic [22], anticancer [23], anti-inflammatory [24], antiviral [25], and antibacterial [26] effects. Interestingly, flavonoid compounds benefited humans in overcoming oxidative damage-related diseases such as cancer, atherosclerosis, asthma, neurodegenerative disease like PD and Alzheimer's disease (AD) [27]. In this review, the current literature on the protective mechanisms of flavonoids in delaying neuronal cell loss in Parkinson's disease is discussed in depth.
2. Activation of Intracellular Antioxidant Enzymes
Several studies have suggested that PD is a consequence of free radicals-induced oxidative stress [28–30]. In a normal state, free radicals are usually detoxified by various internal antioxidant enzymes to less toxic molecules, which are then removed by various ways [31]. However, the natural antioxidant defense systems may not be able to support overwhelming production of free radicals and this could result in a reduction in the activity of these enzymes [23]. Hence, increased free radicals and oxidative stress in cells can culminate in damaging biological molecules including DNA, proteins, and carbohydrates and cell death [32]. The antioxidant enzymes are including superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) that facilitate reactions that help to catalyze the ROS to less toxic molecules [33] (Table 2), thereby playing a key role in preventing lipid peroxidation [23, 24].
Table 2.
| Antioxidant enzyme | Function | Chemical reaction |
|
| ||
| Superoxide dismutase | Catalysing superoxide anion to oxygen and hydrogen peroxide | 2O2 • + 2H+ → H2O2 + O2 |
|
| ||
| Catalase | Detoxifying hydrogen peroxide to water and oxygen molecule | 2H2O2 → O2 + 2H2O |
|
| ||
| Glutathione (GSH) | Electron donor to GPx in reducing hydroperoxides to water molecules | 2GSH + H2O2 → GS–SG + 2H2O |
|
| ||
| Glutathione peroxidase | Reducing hydroperoxides to water molecules | 2GSH + H2O2→ GS–SG + 2H2O |
|
| ||
| Glutathione reductase | Catalyzing the reduction of glutathione disulfide (GSSG) to the sulfhydryl form glutathione (GSH) | GSSG + NADPH + H+ → 2GSH + NADP+ |
O 2 • (superoxide anion); H 2 O 2 (hydrogen peroxide); O 2 (oxygen); H 2 O (water molecule); GSSG (reduced glutathione); NADPH (nicotinamide adenine dinucleotide phosphate).
Flavonoid compounds have been found to activate the endogenous antioxidant status in neuronal cells hence protecting them from undergoing neurodegeneration [31]. Polyphenols such as quercetin glycosides, rutin, and isoquercitrin (Table 3) have distinct features in upregulating the production of intracellular antioxidant enzymes such as SOD, GPx, CAT, and glutathione in a 6-hydroxydopamine- (6-OHDA-) induced in PC-12, rat pheochromocytoma cells [31, 34, 35]. Besides that, quercetin, fisetin, methyl gallate, and propyl gallate were also found to protect neuronal cells from oxidative stress through elevation of intracellular glutathione level [20]. Apart from that, the neuroprotective effects of polyphenols in protecting neuronal cells were further demonstrated in animal studies using naringin [36]. In this study, naringin was found to suppress the 3-nitropropionic acid-induced neuronal apoptosis via activation of SOD, GPX, CAT, and GR (glutathione reductase) in both striatum and plasma of Wistar rats [36]. Genistein [37] and naringenin [38] also have proved to elevate the antioxidant enzymes, namely, superoxide dismutase, and glutathione peroxidase. Although there are numerous findings on the activation of antioxidant enzymes by flavonoids polyphenols, the mechanisms of action is still unclear.
Table 3.
| Types of polyphenol | Studied model: cell or animal | Outcome | References |
|---|---|---|---|
| Apigenin | BV-2 murine microglia cell line and cerebral artery occlusion-induced focal ischemia in mice | (i) Inhibiting production of nitric oxide and prostaglandin E2 (ii) Suppressing p38 mitogen-activated protein kinase (MAPK), c-Jun N-terminal kinase (JNK) phosphorylation (iii) Protecting neuronal cells from injury in middle cerebral artery occlusion |
Ha et al., 2008 [39] |
|
| |||
| Luteolin | Lipopolysaccharide (LPS) induced primary mesencephalic neuron-glia | (i) Attenuating the decrease in dopamine uptake and loss of tyrosine hydroxylase (ii) Inhibiting activation of microglia and excessive production of tumor necrosis factor-α, nitric oxide, and superoxide |
Chen et al., 2008 [40] |
|
| |||
| Kaempferol | Rotenone-induced SH-SY5Y cells and primary neurons | (i) Enhancing mitochondrial turnover by autophagy | Filomeni et al., 2012 [41] |
|
| |||
| Myricetin | MPP+-treated MES23.5 cells | (i) Attenuating cell loss and nuclear condensation (ii) Suppressing the production of intracellular reactive oxygen species (ROS) (iii) Restoring the mitochondrial transmembrane potential (iv) Increasing Bcl-2/Bax ratio and decreasing Caspase 3 activation (v) Decreasing phosphorylation of MAPK kinase 4 and JNK |
Zhang et al., 2011 [42] |
|
| |||
| Quercetin | Rotenone-induced rats | (i) Reducing cell loss in striatal dopamine (ii) Scavenging hydroxyl radicals (iii) Upregulating mitochondrial complex-I activity |
Karuppagounder et al., 2013 [43] |
|
| |||
| Rutin | 6-OHDA induced PC-12 neuronal cells | (i) Activating antioxidant enzymes (SOD, CAT, GPx, GSH) (ii) Suppressing lipid peroxidation |
Magalingam et al., 2013 [31] |
|
| |||
| Isoquercitrin | 6-OHDA induced PC-12 neuronal cells | (i) Activating antioxidant enzymes (SOD, CAT, GPx, GSH) (ii) Suppressing lipid peroxidation |
Magalingam et al., 2014 [34] |
|
| |||
| Catechin | 6-OHDA-lesioned rats | (i) Attenuating the increase in rotational behavior (ii) Improving the locomotor activity (iii) Restoring GSH levels, increasing dopamine and DOPAC content |
Teixeira et al., 2013 [44] |
|
| |||
| (−)−Epigallocatechin 3-gallate | Serum deprived human SH-SY5Y neuroblastoma cells | (i) Inducing the levels of beta tubulin IV and tropomyosin 3 (ii) Increasing the levels of the binding protein 14-3-3 gamma (iii) Decreasing protein levels and mRNA expression of the beta subunit of the enzyme prolyl 4-hydroxylase (iv) Decreasing protein levels of the immunoglobulin-heavy-chain binding protein and the heat shock protein 90 beta |
Weinreb et al., 2007 [45] |
|
| |||
| Hesperidin | 6-OHDA induced aged mice | (i) Preventing memory impairment (ii) Attenuating reduction in GPx and CAT activity, total reactive antioxidant potential, and the DA and its metabolite levels in the striatum (iii) Attenuating reactive species levels and glutathione reductase |
Antunes et al., 2014 [46] |
|
| |||
| Fisetin | lipopolysaccharide (LPS) stimulated BV-2 microglia cells | (i) Suppressing the production of TNF-α, nitric oxide, and PG E2
(ii) Inhibiting the gene expression of TNF-α, interleukin (IL-1β), COX-2, and (iNOS) at both mRNA and protein levels. (iii) Suppressing IκB degradation, nuclear translocation of NF-κB, and phosphorylation of p38 MAPKs |
Zheng et al., 2008 [47] |
|
| |||
| Naringenin | 6-OHDA induced SH-SY5Y cells and mice | (i) Increasing in nuclear factor E2-related factor 2 (Nrf2) protein levels and activating of antioxidant response pathway genes (ii) Protecting nigrostriatal dopaminergic neurons against neurodegeneration and oxidative damage |
Lou et al., 2014 [48] |
|
| |||
| Theaflavin | MPTP-induced mouse | (i) Reducing oxidative stress (ii) Improving motor behavior and expression of dopamine transporter and vesicular monoamine transporter 2 in striatum and substantia nigra. |
Anandhan et al., 2012 [49] |
|
| |||
| Proanthocyanidin | Rotenone in a primary neuronal cell | (i) Protecting dopaminergic cell (ii) Rescuing mitochondrial respiration in a dopaminergic cell line |
Strathearn et al., 2014 [50] |
Several studies have investigated the correlation between free radicals and antioxidant enzymes and a vast number of findings have shown increase in free radical-induced oxidative damage in in vitro test systems to be indirectly proportional to the activation of internal antioxidant enzymes [31]. Intriguingly, flavonoids caused activation of these antioxidant enzymes in free radical-induced test systems [31]. Along with this, the activation of intracellular enzymes can be explained by two mechanisms; (i) flavonoids attenuated the free radicals-induced damage on antioxidant enzymes by scavenging the radicals [51] and (ii) flavonoids bound with the antioxidant enzymes and caused direct activation of these enzymes, where any of these mechanisms will result in increased activity of the antioxidant enzyme [31]. In one of the earlier discoveries, Nagata and coworkers (1999) have shown that the antioxidant effects of quercetin and catechin are mediated by direct interaction with the GPx enzyme [52]. These flavonoids cause modulation in the structure-activity of GPx and thereby enhanced its antioxidant activity [52, 53]. In a similar study, it was found that addition of rutin, quercitrin, myricetin, and kaempferol to catalase had a direct effect of activation of catalase and this effect was attributed to the binding of these polyphenols to heme moiety or protein region of this enzyme [54].
3. Suppression of Lipid Peroxidation in Parkinson's Disease
Free radicals and ROS are generated as by-products of several normal cellular functions such as the mitochondrial oxidative phosphorylation system, phagocytosis, and the arachidonic acid metabolism pathway [55]. Most ROS such as the superoxide anion (O2 •), hydrogen peroxide (H2O2), nitrogen species (NO), hydroxyl (OH−), and alkoxyl radicals are hazardous to cells until it is well catabolized to its less toxic substance by the natural antioxidant enzyme systems. However, if the consistent build-up of ROS and free radicals cannot be supported by the various antioxidant enzyme systems, these conditions can result in oxidative stress and lipid peroxidation, which eventually lead to cellular damage. Several studies have suggested that the presence of neurotoxic substances in the human brain may augment the ROS-induced oxidative damage [56–58]. For instance, in PD, prolonged exposure to neurotoxins such as paraquat and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) leads to increased generation of ROS in brain neurons as these toxic substances could not be effectively removed by the natural antioxidant enzymes in the brain. This, in turn, inhibited the mitochondrial complex I system, oxidation of polyunsaturated fatty acid (PUFA), protein aggregation, and DNA damage in the neuronal cells [59].
Pryor and Porter were the pioneers in suggesting that lipid peroxidation of certain polyunsaturated fatty acids (PUFA) produces 4-hydroxy-2-nonenal (HNE) as one of the many by-products [60]. HNE is an interesting by-product as it is cytotoxic and appears to be involved in various degenerative diseases, including diabetes [61], pulmonary diseases [62], and Parkinson's disease [63]. In Parkinson's disease, for instance, HNE is found to be an effective protein modifier that induces cross-linking of the monomeric α-synuclein molecules, thereby converting these proteins into high molecular weight β-sheet-rich oligomers [63, 64]. The α-synuclein is a soluble protein consisting of 140 amino acid molecules and is usually located at the presynaptic regions of neurons. Several studies have suggested that α-synuclein is involved in neurotransmitter secretion as well as in the regulation of synaptic vesicle pool and plasticity [65–67]. In the pathogenesis of PD, it has been proposed that oxidative stress triggers a vicious cycle by inducing lipid peroxidation and accumulation of α-synuclein aggregates, which forms Lewy bodies, which forms Lewy bodies, which are associated with neuronal dysfunction that triggers the onset of PD symptoms [68, 69].
There is a substantial body of evidence, which suggest that flavonoid-rich cocoa-derived foods possess free radicals scavenging property against the superoxide anions such as H2O2, HClO, and peroxynitrite [70, 71]. The flavan-3-ol compounds present in cocoa are the monomers catechin and epicatechin and the dimer procyanidin B2. These compounds were shown to inhibit lipid peroxidation in brain homogenates and human plasma via anon-enzymatic system [70, 72]. Inhibition of lipid peroxidation in neuronal cells could help to delay the ongoing neurodegeneration process in PD [73]. Besides that, quercetin glycoside derivatives, rutin, and isoquercitrin have shown potent antioxidant potential by attenuating lipid peroxidation induced by 6-OHDA on PC12 neuronal cells (Table 3) [31, 34, 35]. In addition, black tea extract, which contains epigallocatechin (EGCG) polyphenol, was also reported to suppress lipid peroxidation in a 6-OHDA induced rat model of PD [74, 75]. The black tea pretreated rats showed attenuation of lipid peroxidation by 59% compared to the rats in the control group, which were only treated with 6-OHDA [75]. The polyphenol theaflavin was reported to inhibit xanthine oxidase (XO), an enzyme involved in producing superoxides, hence protecting the neuronal cells from undergoing lipid peroxidation [76]. The common feature of most polyphenols, that is, their antioxidant property, is only evident during oxidative stress condition and is not usually demonstrable under normal condition [75]. These convincing evidences markedly support the ability of flavonoids to exert neuroprotective roles via scavenging ROS generated during oxidative stress and subsequently suppress lipid peroxidation in neuronal cells or in animal models of PD.
4. Inhibition of Proinflammatory and Proapoptotic Mediators
Several lines of evidence suggest that microglia activation has a close association with the pathogenesis of PD [77, 78]. This is in line with the discovery of high levels of microglia activation in the vicinity of degenerating dopaminergic neurons and other areas of the human brain such as hippocampus, cingulate cortex, and temporal cortex [79, 80]. Microglia activation is initiated by the presence of extracellular stimuli including endotoxin, cytokines, misfolded or damaged proteins, and chemokines [81]. In the event of microglial activation, the redox-sensitive nuclear factor-kappa-B (NF-κB) found in the cytoplasm will translocate to the nuclear compartment of the cell and form adducts with the DNA. This results in the activation of various proinflammatory genes such as interleukin-1 beta (IL-1β), tumor necrosis factor-alpha (TNF-α), cyclooxygenase-2 (COX-2), and inducible nitric oxide synthase (iNOS) as well as IL-6 (Figure 1) [82, 83].
Figure 1.
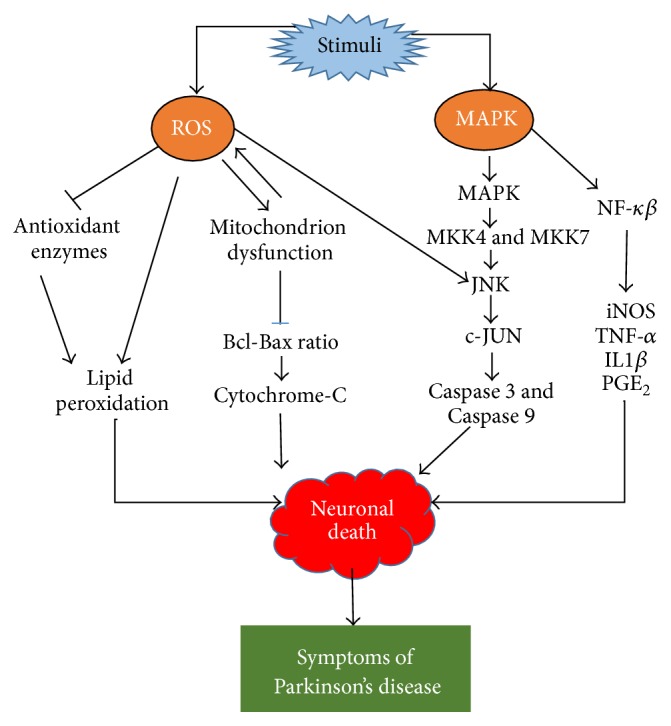
Simplified depiction of ROS and MAPK-induced cytotoxicity. External stimuli including neurotoxin or lipopolysaccharide could generate ROS that is able to suppress the endogenous antioxidant enzymes particularly superoxide dismutase, glutathione peroxidase, and catalase and leads to increase in lipid peroxidation and cell death. The ROS has the ability to directly cause lipid peroxidation and cellular damage as well affecting the mitochondria metabolism, which suppresses the Bcl-Bax ratio and result in leakage of cytochrome-c from mitochondria and eventually cell death. The presence of external stimuli activates MAPK-induced inflammatory mediators including JNK and c-JUN that cause activation of proapoptotic caspases, namely, Caspase 3 and Caspase 9; and the effect is cellular apoptosis. The MAPK family is also responsible in initiating the NF-κB induced expression of proinflammatory cytokine genes (iNOS, TNF-α, and ILIβ). The symptoms of Parkinson's disease occur as a result of neurodegeneration of dopamine producing neurons.
Some studies have found neuroinflammation to be associated with the pathogenesis of PD. Hence, a host of anti-inflammatory therapies have been tested using cell-based and rat models of PD to test the ability of various neuroinflammatory mediators such as Dexamethasone [84], aspirin [85], interleukin-10 [86], and Minocycline [87] to suppress the onset of Parkinson-like symptoms. However, one potential approach to ameliorate the neuroinflammation process is by applying natural polyphenols as the therapeutic agent, since these compounds do not have any significant known adverse effects and they appear to promise almost similar outcomes as conventional drug therapies [88]. The potency of various polyphenols tested using both cell-based and animal model of PD is summarized in Table 3. Various flavonoids such as genistein [89], morin [90], kaempferol [91], and emodin [92] were reported to suppress secretion of TNF-α. A recent study found decreased expression of NF-κB, iNOS, and COX-2 genes in naringenin pretreated Wistar rats in an experimental model of focal cerebral ischemia/reperfusion (I/R) induced inflammation [83]. In this study, naringenin upregulated the antioxidant status in the naringenin-treated rats as well as inhibiting the expression of NF-κB and activation of downstream genes that can trigger the inflammation cascade [83]. Apigenin has gained particular attention as an anti-inflammatory agent that inhibit the expression of nitric oxide (NO), iNOS, and COX-2 in lipopolysaccharide- (LPS-) induced RAW 264.7 cells [93].
Interestingly, there are also studies that show that flavonol polyphenol myricetin can increase the Bcl/Bax ratio as well as decreasing Caspase 3 expression in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPP+) induced cell model of PD (Figure 1) [94]. The Bcl-2 family is associated with mitochondrial function and plays a pivotal role in the activation of caspases and apoptosis [95]. The Bcl-2 protein is an antiapoptotic agent that binds to Bax, which decreases the proapoptotic effect of Bax by forming Bcl/Bax heterodimers, a preset ratio that determines the survival or death of cells following an apoptotic stimulus [96]. Hence, an increase in the Bcl/Bax ratio by myricetin pretreatment increased the survival of the neuronal cells [42]. Genistein, an isoflavone, largely found in Soy bean has been shown to possess antiapoptotic effects in 6-OHDA induced SK-N-SH human neuroblastoma cells, where genistein was found to attenuate upregulation of Bax induced by exposure 6-OHDA as well as downregulating the expression of Bcl-2 mRNA and protein [97].
Flavonoids can also exert its protective mechanism via modulation of the mitogen-activated protein kinase (MAPK) signaling pathways [98]. During oxidative stress, the MAPK pathways are activated, ensuing phosphorylation of MAPK kinase 4 (MKK4), a unique protein among the MKK family that is widely distributed in rat and human brains, particularly in the cerebral cortex, hypothalamus, and hippocampus [99]. The stress-induced phosphorylation of the MKK4 results in activation of extracellular signal-regulated kinase 5 (ERK5), c-Jun N-terminal kinase (JNK), and p38 that activates downstream proinflammatory mediators (Figure 1) [100]. Apigenin was found to protect neuronal cells via suppressing the phosphorylation of p38, MAPK, and JNK but not the ERK pathway [93]. A similar finding was observed with theaflavins and thearubigins, the major polyphenols of black tea, whereby a sustained activation of the p38 MAPK and JNK were observed but not in the ERK pathway [101]. Moreover, cocoa procyanidin binds directly to MKK4, inhibiting its activity and also suppressing the JNK signaling pathway [102]. In most of these studies, polyphenols have been reported to attenuate the proinflammatory and proapoptotic mediators and protect the neuronal cells.
5. Mitochondria Targeted Flavonoid Therapy
Mitochondria, a defined cytoplasmic organelle, plays a pivotal in cellular aerobic respiration and regulation of Ca2+ homeostasis and is also involved in orchestration of cellular apoptosis and production of ROS [103, 104]. Dysfunctions in the physiological processes of mitochondria can lead to the onset of age-related diseases such as PD and AD [104]. In line with recent understanding, mitochondrial dysfunction can be caused by deficiency in Complex I, which plays a crucial role in mitochondria respiration chain [105]. Exposure of neuronal cells to neurotoxins such as MPP+, 6-OHDA, and paraquat causes selective uptake of these toxins by the dopaminergic neurons where these toxins inhibit the activity of Complex I [106]. The decrease in Complex I activity produces excess superoxide radicals that are capable of overwhelming the natural antioxidant systems and eventually cause oxidative stress and neurodegeneration [107]. Several studies have shown mitochondrial ROS production in cells to be the most important source of ROS despite other sources such as nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) [108], XO, cytochrome P450, and the mitochondrial electron transport chain (ETC) [109]. The main reason for mitochondrial ROS to garner mounting attention is its ability to directly activate mediators of proinflammatory cytokine and MAPK [110], which can lead to several pathological conditions such as cancers, cardiovascular, and neurodegenerative diseases.
Mitochondrial dysfunction can also be caused by the accumulation and aggregation of amyloid-beta (Aβ) peptides [111]. Occurrence of Aβ peptides in neuronal cell is a pathological hallmark in neurodegenerative diseases, particularly PD and AD [112]. A recent study has proposed that soluble amyloid aggregates that are formed in neuronal cells have the inherent capacity to penetrate the mitochondrial membrane and induce neuronal death [113]. The penetration of the Aβ peptides occurs through the mitochondria-associated endoplasmic reticulum membranes, which is a physical connection between the membrane of the endoplasmic reticulum and the mitochondrial outer membrane [113]. Hence, mitochondria targeted polyphenol therapy is an excellent approach in modulating mitochondrial dynamic, function, and biogenesis [114].
Quercetin polyphenol is one of the flavonoids compounds that is widely investigated and reported for its antioxidant [115], anticancer [116], anti-inflammatory [117], and antiviral [118] effects. Interestingly, a recent study has shown that quercetin has the ability to repair the mitochondrial electron transport defect in a rotenone-induced rat model of Parkinsonism (Table 2) [43]. This study also demonstrated a dose-dependent upregulation of Complex I activity in the mitochondria, which the authors attribute to the powerful hydroxyl radicals scavenging action of quercetin [43]. In another study, EGCG, a natural polyphenol derived from green tea, was reported to restore mitochondrial energy deficit in lymphoblasts and fibroblasts from Down syndrome patients [119]. The protective mechanism of EGCG is not clearly defined, but it is proposed that Complex I activity and ATP synthase catalytic activities have been activated beside promotion of cellular levels of cyclic adenosine monophosphate (cAMP) and protein kinase A (PKA) dependent phosphorylation of Complex I. Treatment with EGCG effectively stimulated mitochondrial biogenesis in the lymphoblasts and fibroblasts Down syndrome patients via activation of the Sirtuin 1 (SIRT1) dependent Peroxisome proliferator-activated receptor-γ coactivator (PGC-1α), nuclear respiratory factor-1 (NRF-1), and mitochondrial DNA content [119]. There is also accumulating evidence that supports the protective effect of genistein on neuronal cells against oxidative damage [120] and glutamate and Aβ amyloid toxicity [121]. Furthermore, it has been reported that genistein exerts its protective mechanism via restoring mitochondrial membrane potential that was significantly decreased by 6-OHDA treatment in SK-N-SH neuroblastoma cells [97]. Naringin, a ubiquitously found flavanone glycoside, has been reported to exhibit several protective effects including antioxidant, ROS scavenging, and metal chelating activities [122–124]. Besides improving cognitive dysfunction and oxidative defense, it was reported that naringin can restore mitochondrial enzyme functions, specifically Complexes I and III activity in a murine model [38].
6. Modulation of Gene Expression Changes
Neuronal cells that undergo a programmed cell death or apoptosis are regulated by both “protective” and “destructive” genes. “Protective genes” are genes that execute protective mechanism by suppressing oxidative stress, thereby protecting cells, such as thioredoxin reductase-1, glutathione S-transferase, pi 2 (Gstp2), superoxide dismutase (SOD2), copper chaperone for SOD1 (CCS), glucose-6-phosphate dehydrogenase (G6PD), and Bcl-2 [125]. In contrast, increased expression of certain genes such as neuronal cell death-inducible putative kinase (NIPK), ankyrin repeat domain-3 (Ankrd3), protein phosphatase 1G (Ppm1g), and ubiquitin carboxyl-terminal hydrolase-20 results in cellular death in PC-12 cells following exposure to 6-OHDA [125]. Studies on PD model showed that treatment of dopaminergic cells (e.g., PC12 cells) with neurotoxins like 6-OHDA or MPTP upregulated proapoptotic genes and other “destructive” genes that promote cellular apoptosis [125]. Hence, therapeutic drug targeting these “destructive” genes may protect neurons from undergoing apoptotic process and neuronal death.
In our previous study, we have shown that quercetin glycosides, rutin, and isoquercitrin induced neuroprotection by changes in gene expression in 6-OHDA treated PC-12 rat pheochromocytoma cells [126]. Rutin pretreatment attenuated the expression of Parkin 5 (Park 5), Parkin 7 (Park 7), Caspase 3, Caspase 7, and Ataxin 2 gene expression that were highly expressed by 6-OHDA. Moreover, rutin upregulated the protective genes, including tyrosine hydroxylase (Th), neuron specific gene family member 1 (Nsg1), N-ethylmaleimide-sensitive factor (Nsf), and optic atrophy 1 homolog (Opa1) genes [126]. On the hand, isoquercitrin suppressed Park 5 and Park 7 genes and stimulated Nsf and Nsg1 genes [126] A recent study found that quercetin inhibited NO production by suppressing inducible the transcription of the iNOS gene [127]. Quercetin reportedly exerted this effect by suppressing the signaling pathway that leads to the activation of NF-κB, activating protein-1 (AP-1) and signal transducer, and activator of transcription-1 (STAT1), which are the key intracellular agents that contribute to the neuroinflammatory process [128]. This study also found that quercetin upregulated the expression of the heme oxygenase-1 gene in the BV-2 microglia cells [128]. The heme oxygenase-1 was recently named the “therapeutic funnel” as it was found to possess anti-inflammatory and antiapoptotic effects [129]. In addition, quercetin-induced heme oxygenase 1 gene expression was reported to be related to the activation of tyrosine kinase and MAPK [127].
Emerging evidence suggests that genistein, an isoflavone naturally present in Soy beans, reduced MPTP-induced neurotoxicity in a murine model of PD via activation of the Bcl-2 mRNA level in the midbrain [130]. Bcl-2 is an antiapoptotic regulatory protein that maintains mitochondrial integrity by inhibiting the release of cytochrome-c and the intrinsic cascade that leads to activation of Caspase 3 and apoptosis [131]. The neuroprotective mechanism of genistein was attributed to its increased affinity towards the estrogen receptor (ER) hence affecting estrogen-regulated Bcl-2 gene expression [130]. Previous studies have shown that estradiol treatment could stimulate Bcl-2 expression in the hypothalamus [132], cerebral cortex [133] as well as neuronal cell line [134]. Therefore, isoflavone genistein could be an effective neuroprotective therapy to reduce the neurotoxin-induced dopaminergic neuronal loss and increase cell survival in human midbrain. Amongst different flavonoids, epigallocatechin-3-gallate (ECGC) was found to modulate changes in gene expression in neuronal cell cultures [135]. Exposure to EGCG was reported to attenuate 6-OHDA induced neuronal loss by preventing the expression of proapoptotic genes such as Bax, Bad, and Mdm2 and decrease the expression of antiapoptotic gene expression including Bcl-2, Bcl-w, and Bcl-x(l) [135].
7. Summary
Polyphenol flavonoids are found ubiquitously in a wide range of fruits and vegetables such as apple skin, celery, oranges, onion, mango, apples, and buckwheat, as well as food and beverages derived from plants including olive oil, black/green tea, and red wine. Over the last two decades, a significant amount of data pertaining to the antioxidant effects of different types of flavonoids has been documented. Studies to validate neuroprotective effects of flavonoids were performed induced neurotoxins with either pre- or posttreatment with flavonoid compounds based on the objective of the study. Almost all the published literature suggested that flavonoids can exert neuroprotective effects in pathological conditions, that is, in the presence of prooxidants or neurotoxins but not under normal physiological conditions. These findings clearly explain the antioxidant nature of flavonoids in arresting free radical-induced oxidative damage, which is known to be central to many degenerating diseases including PD. Various types of flavonoid were tested in many types of disease model in both in vitro and in vivo experimental set-ups. Some of the many protective effects of flavonoids reported included antiapoptosis, antibacterial, antiviral, antioxidant, anticancer, antidiabetic, and anti-inflammatory. However, in terms of neuroprotection, the antiapoptotic and anti-inflammatory ability of flavonoids appear to impede the progressive neuronal loss in neurodegenerative diseases particularly PD. Apart from that, flavonoids such as quercetin, rutin, isoquercitrin, and catechin were found to increase the levels of the natural antioxidant enzymes in the cellular compartment as a bid to suppress the free radical-induced lipid peroxidation. Besides that, flavonoids were also found to downregulate the neuroinflammation process by inhibiting the MAPK signaling pathways that can attenuate the activation of the ERK5, JNK, and p38 signalling pathways, which stimulate production of more downstream proinflammatory mediators. In addition, EGCG was found to modulate expression of proapoptotic genes like Bax, Bad, and Mdm2 whilst genistein induced changes in the expression of the Bcl-2 gene, thereby increasing survival of cells in neurodegenerative diseases. Although flavonoids have shed some light as neuroprotective agents, there are many barrels and barricades in this area of research. To date, the pathogenesis of PD is still poorly defined as the main trigger of the dopaminergic neuronal loss is still largely unknown, although some of the main contributing risk factors like genetic predisposition, environmental toxins, and lack of exercise have been identified. Elucidation of the pathogenesis of PD will further aid in the search to identify flavonoid compounds to stop the trigger point of the “domino” cascade of events involved in neuronal cell death.
8. Future Perspectives
The design of the “magic bullet” as a therapeutic approach to help either prevent or treat PD depends on our understanding of the mechanisms by which flavonoids counteract neuronal damage. Although some mechanisms have been described well, we are still far from getting the complete picture of protective mechanism of flavonoid polyphenol. There are many loop holes in the comprehension of the mechanism by which flavonoids protect neuronal cell; for instance, (i) studies evaluating flavonoids to promote neuronal function and neurite outgrowth in human dopaminergic neurons are limited; (ii) clinical trials of neuroprotection evidence of most promising flavonoid polyphenols in PD patient are scarce; and (iii) strategies to introduce flavonoids and therapeutic dosage as these molecules change compositions in in vivo system upon exposure to the acidic environment of gastric cavity and finally studies evaluating the ability of emerging flavonoids compounds to cross the blood brain barrier are needed as very few flavonoids have been tested for this ability in animal models.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Corti O., Hampe C., Darios F., Ibanez P., Ruberg M., Brice A. Parkinson's disease: from causes to mechanisms. Comptes Rendus Biologies. 2005;328(2):131–142. doi: 10.1016/j.crvi.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Shimohama S., Sawada H., Kitamura Y., Taniguchi T. Disease model: Parkinson's disease. Trends in Molecular Medicine. 2003;9(8):360–365. doi: 10.1016/s1471-4914(03)00117-5. [DOI] [PubMed] [Google Scholar]
- 3.Double K. L. Neuronal vulnerability in Parkinson's disease. Parkinsonism and Related Disorders. 2012;18(supplement 1):S52–S54. doi: 10.1016/S1353-8020(11)70018-9. [DOI] [PubMed] [Google Scholar]
- 4.Nikam S., Nikam P., Ahaley S. K., Sontakke A. V. Oxidative stress in Parkinson's disease. Indian Journal of Clinical Biochemistry. 2009;24(1):98–101. doi: 10.1007/s12291-009-0017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blum D., Torch S., Lambeng N., et al. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson's disease. Progress in Neurobiology. 2001;65(2):135–172. doi: 10.1016/s0301-0082(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 6.Schapira A. H. V. Etiology of Parkinson's disease. Neurology. 2006;66(10, supplement 4):S10–S23. doi: 10.1212/wnl.66.10_suppl_4.s10. [DOI] [PubMed] [Google Scholar]
- 7.Huang Z., De la Fuente-Fernández R., Stoessl A. J. Etiology of Parkinson's disease. Canadian Journal of Neurological Sciences. 2003;30(1):S10–S18. doi: 10.1017/S031716710000319X. [DOI] [PubMed] [Google Scholar]
- 8.Hald A., Lotharius J. Oxidative stress and inflammation in Parkinson's disease: is there a causal link? Experimental Neurology. 2005;193(2):279–290. doi: 10.1016/j.expneurol.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Walker R. W., Hand A., Jones C., Wood B. H., Gray W. K. The prevalence of Parkinson's disease in a rural area of North-East England. Parkinsonism and Related Disorders. 2010;16(9):572–575. doi: 10.1016/j.parkreldis.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Freire C., Koifman S. Pesticide exposure and Parkinson's disease: epidemiological evidence of association. NeuroToxicology. 2012;33(5):947–971. doi: 10.1016/j.neuro.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Gatto N. M., Cockburn M., Bronstein J., Manthripragada A. D., Ritz B. Well-water consumption and Parkinson's disease in rural California. Environmental Health Perspectives. 2009;117(12):1912–1918. doi: 10.1289/ehp.0900852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai B. C. L., Marion S. A., Teschke K., Tsui J. K. C. Occupational and environmental risk factors for Parkinson's disease. Parkinsonism and Related Disorders. 2002;8(5):297–309. doi: 10.1016/S1353-8020(01)00054-2. [DOI] [PubMed] [Google Scholar]
- 13.Prakash K. M., Tan E. K. Clinical evidence linking coffee and tea intake with Parkinson's disease. Basal Ganglia. 2011;1(3):127–130. doi: 10.1016/j.baga.2011.07.001. [DOI] [Google Scholar]
- 14.Hong D.-P., Fink A. L., Uversky V. N. Smoking and Parkinson's disease: does nicotine affect α-synuclein fibrillation? Biochimica et Biophysica Acta—Proteins and Proteomics. 2009;1794(2):282–290. doi: 10.1016/j.bbapap.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Q., Park Y., Huang X., et al. Physical activities and future risk of Parkinson disease. Neurology. 2010;75(4):341–348. doi: 10.1212/WNL.0b013e3181ea1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerecke K. M., Jiao Y., Pani A., Pagala V., Smeyne R. J. Exercise protects against MPTP-induced neurotoxicity in mice. Brain Research. 2010;1341:72–83. doi: 10.1016/j.brainres.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao L. H., Jiang Y. M., Shi J., et al. Flavonoids in food and their health benefits. Plant Foods for Human Nutrition. 2004;59(3):113–122. doi: 10.1007/s11130-004-0049-7. [DOI] [PubMed] [Google Scholar]
- 18.Schmitt-Schillig S., Schaffer S., Weber C. C., Eckert G. P., Müller W. E. Flavonoids and the aging brain. Journal of Physiology and Pharmacology. 2005;56(1):23–36. [PubMed] [Google Scholar]
- 19.Ebrahimi A., Schluesener H. Natural polyphenols against neurodegenerative disorders: potentials and pitfalls. Ageing Research Reviews. 2012;11(2):329–345. doi: 10.1016/j.arr.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Ishige K., Schubert D., Sagara Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radical Biology and Medicine. 2001;30(4):433–446. doi: 10.1016/S0891-5849(00)00498-6. [DOI] [PubMed] [Google Scholar]
- 21.Pietta P. G. Flavonoids as antioxidants. Journal of Natural Products. 2000;63(7):1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 22.Niki E. Do antioxidants impair signaling by reactive oxygen species and lipid oxidation products? FEBS Letters. 2012;586(21):3767–3770. doi: 10.1016/j.febslet.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 23.Li C., Zhou H.-M. The role of manganese superoxide dismutase in inflammation defense. Enzyme Research. 2011;2011:6. doi: 10.4061/2011/387176.387176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sackesen C., Ercan H., Dizdar E., et al. A comprehensive evaluation of the enzymatic and nonenzymatic antioxidant systems in childhood asthma. Journal of Allergy and Clinical Immunology. 2008;122(1):78–85. doi: 10.1016/j.jaci.2008.03.035. [DOI] [PubMed] [Google Scholar]
- 25.Pasupathi P., Chandrasekar V., Kumar U. S. Evaluation of oxidative stress, enzymatic and non-enzymatic antioxidants and metabolic thyroid hormone status in patients with diabetes mellitus. Diabetes & Metabolic Syndrome: Clinical Research and Reviews. 2009;3(3):160–165. doi: 10.1016/j.dsx.2009.07.004. [DOI] [Google Scholar]
- 26.Harish G., Venkateshappa C., Mythri R. B., et al. Bioconjugates of curcumin display improved protection against glutathione depletion mediated oxidative stress in a dopaminergic neuronal cell line: implications for Parkinson's disease. Bioorganic and Medicinal Chemistry. 2010;18(7):2631–2638. doi: 10.1016/j.bmc.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 27.Unnikrishnan M. K., Veerapur V., Nayak Y., Mudgal P. P., Mathew G. Polyphenols in Human Health and Disease. chapter 13. Vol. 1. Elsevier; 2014. Antidiabetic, antihyperlipidemic and antioxidant effects of the flavonoids; pp. 143–161. [DOI] [Google Scholar]
- 28.Koutsilieri E., Scheller C., Grünblatt E., Nara K., Li J., Riederer P. Free radicals in Parkinson's disease. Journal of Neurology. 2002;249(supplement 2):II1–II5. doi: 10.1007/s00415-002-1201-7. [DOI] [PubMed] [Google Scholar]
- 29.Kumar H., Lim H.-W., More S. V., et al. The role of free radicals in the aging brain and Parkinson's disease: convergence and parallelism. International Journal of Molecular Sciences. 2012;13(8):10478–10504. doi: 10.3390/ijms130810478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams J. D., Jr., Odunze I. N. Oxygen free radicals and Parkinson's disease. Free Radical Biology and Medicine. 1991;10(2):161–169. doi: 10.1016/0891-5849(91)90009-R. [DOI] [PubMed] [Google Scholar]
- 31.Magalingam K. B., Radhakrishnan A., Haleagrahara N. Rutin, a bioflavonoid antioxidant protects rat pheochromocytoma (PC-12) cells against 6-hydroxydopamine (6-OHDA)-induced neurotoxicity. International Journal of Molecular Medicine. 2013;32(1):235–240. doi: 10.3892/ijmm.2013.1375. [DOI] [PubMed] [Google Scholar]
- 32.Floyd R. A., Carney J. M. Free radical damage to protein and DNA: mechanisms involved and relevant observations on brain undergoing oxidative stress. Annals of Neurology. 1992;32:S22–S27. doi: 10.1002/ana.410320706. [DOI] [PubMed] [Google Scholar]
- 33.Sun Y. Free radicals, antioxidant enzymes, and carcinogenesis. Free Radical Biology and Medicine. 1990;8(6):583–599. doi: 10.1016/0891-5849(90)90156-D. [DOI] [PubMed] [Google Scholar]
- 34.Magalingam K. B., Radhakrishnan A., Haleagrahara N. Protective effects of flavonol isoquercitrin, against 6-hydroxydopamine (6-OHDA)—induced toxicity in PC12 cells. BMC Research Notes. 2014;7(1, article 49) doi: 10.1186/1756-0500-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang J., Guo J., Yuan J. In vitro antioxidant properties of rutin. LWT—Food Science and Technology. 2008;41(6):1060–1066. doi: 10.1016/j.lwt.2007.06.010. [DOI] [Google Scholar]
- 36.Gopinath K., Prakash D., Sudhandiran G. Neuroprotective effect of naringin, a dietary flavonoid against 3-Nitropropionic acid-induced neuronal apoptosis. Neurochemistry International. 2011;59(7):1066–1073. doi: 10.1016/j.neuint.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 37.Qian Y., Guan T., Huang M., et al. Neuroprotection by the soy isoflavone, genistein, via inhibition of mitochondria-dependent apoptosis pathways and reactive oxygen induced-NF-κB activation in a cerebral ischemia mouse model. Neurochemistry International. 2012;60(8):759–767. doi: 10.1016/j.neuint.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 38.Kumar A., Prakash A., Dogra S. Naringin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress induced by d-galactose in mice. Food and Chemical Toxicology. 2010;48(2):626–632. doi: 10.1016/j.fct.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 39.Ha S. K., Lee P., Park J. A., et al. Apigenin inhibits the production of NO and PGE2 in microglia and inhibits neuronal cell death in a middle cerebral artery occlusion-induced focal ischemia mice model. Neurochemistry International. 2008;52(4-5):878–886. doi: 10.1016/j.neuint.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 40.Chen H.-Q., Jin Z.-Y., Wang X.-J., Xu X.-M., Deng L., Zhao J.-W. Luteolin protects dopaminergic neurons from inflammation-induced injury through inhibition of microglial activation. Neuroscience Letters. 2008;448(2):175–179. doi: 10.1016/j.neulet.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 41.Filomeni G., Graziani I., de Zio D., et al. Neuroprotection of kaempferol by autophagy in models of rotenone-mediated acute toxicity: possible implications for Parkinson's disease. Neurobiology of Aging. 2012;33(4):767–785. doi: 10.1016/j.neurobiolaging.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 42.Zhang K., Ma Z., Wang J., Xie A., Xie J. Myricetin attenuated MPP+-induced cytotoxicity by anti-oxidation and inhibition of MKK4 and JNK activation in MES23.5 cells. Neuropharmacology. 2011;61(1-2):329–335. doi: 10.1016/j.neuropharm.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 43.Karuppagounder S. S., Madathil S. K., Pandey M., Haobam R., Rajamma U., Mohanakumar K. P. Quercetin up-regulates mitochondrial complex-I activity to protect against programmed cell death in rotenone model of Parkinson's disease in rats. Neuroscience. 2013;236:136–148. doi: 10.1016/j.neuroscience.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 44.Teixeira M. D. A., Souza C. M., Menezes A. P. F., et al. Catechin attenuates behavioral neurotoxicity induced by 6-OHDA in rats. Pharmacology Biochemistry and Behavior. 2013;110:1–7. doi: 10.1016/j.pbb.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 45.Weinreb O., Amit T., Youdim M. B. H. A novel approach of proteomics and transcriptomics to study the mechanism of action of the antioxidant–iron chelator green tea polyphenol (-)-epigallocatechin-3-gallate. Free Radical Biology and Medicine. 2007;43(4):546–556. doi: 10.1016/j.freeradbiomed.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 46.Antunes M. S., Goes A. T. R., Boeira S. P., Prigol M., Jesse C. R. Protective effect of hesperidin in a model of Parkinson’s disease induced by 6-hydroxydopamine in aged mice. Nutrition. 2014;30(11-12):1415–1422. doi: 10.1016/j.nut.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 47.Zheng L. T., Ock J., Kwon B.-M., Suk K. Suppressive effects of flavonoid fisetin on lipopolysaccharide-induced microglial activation and neurotoxicity. International Immunopharmacology. 2008;8(3):484–494. doi: 10.1016/j.intimp.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 48.Lou H., Jing X., Wei X., Shi H., Ren D., Zhang X. Naringenin protects against 6-OHDA-induced neurotoxicity via activation of the Nrf2/ARE signaling pathway. Neuropharmacology. 2014;79:380–388. doi: 10.1016/j.neuropharm.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 49.Anandhan A., Tamilselvam K., Radhiga T., Rao S., Essa M. M., Manivasagam T. Theaflavin, a black tea polyphenol, protects nigral dopaminergic neurons against chronic MPTP/probenecid induced Parkinson's disease. Brain Research. 2012;1433:104–113. doi: 10.1016/j.brainres.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 50.Strathearn K. E., Yousef G. G., Grace M. H., et al. Neuroprotective effects of anthocyanin-and proanthocyanidin-rich extractsin cellular models of Parkinson's disease. Brain Research. 2014;1555:60–77. doi: 10.1016/j.brainres.2014.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang S. Y., Ballington J. R. Free radical scavenging capacity and antioxidant enzyme activity in deerberry (Vaccinium stamineum L.) LWT—Food Science and Technology. 2007;40(8):1352–1361. doi: 10.1016/j.lwt.2006.09.005. [DOI] [Google Scholar]
- 52.Nagata H., Takekoshi S., Takagi T., Honma T., Watanabe K. Antioxidative action of flavonoids, quercetin and catechin, mediated by the activation of glutathione peroxidase. Tokai Journal of Experimental and Clinical Medicine. 1999;24(1):1–11. [PubMed] [Google Scholar]
- 53.Zhu J., Zhang X., Li D., Jin J. Probing the binding of flavonoids to catalase by molecular spectroscopy. Journal of Molecular Structure. 2007;843(1–3):38–44. doi: 10.1016/j.molstruc.2006.12.033. [DOI] [Google Scholar]
- 54.Doronicheva N., Yasui H., Sakurai H. Chemical structure-dependent differential effects of flavonoids on the catalase activity as evaluated by a chemiluminescent method. Biological and Pharmaceutical Bulletin. 2007;30(2):213–217. doi: 10.1248/bpb.30.213. [DOI] [PubMed] [Google Scholar]
- 55.Cadenas E., Davies K. J. A. Mitochondrial free radical generation, oxidative stress, and aging. Free Radical Biology & Medicine. 2000;29(3-4):222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 56.Singh R. P., Sharad S., Kapur S. MPTP as a mitochondrial neurotoxic model of parkinson’s disease. free radicals and oxidative stress in neurodegenerative diseases: relevance of dietary antioxidants. Journal, Indian Academy of Clinical Medicine. 2004;5(3):218–225. [Google Scholar]
- 57.Przedborski S., Tieu K., Perier C., Vila M. MPTP as a mitochondrial neurotoxic model of Parkinson's disease. Journal of Bioenergetics and Biomembranes. 2004;36(4):375–379. doi: 10.1023/b:jobb.0000041771.66775.d5. [DOI] [PubMed] [Google Scholar]
- 58.Di Monte D. A. The environment and Parkinson's disease: is the nigrostriatal system preferentially targeted by neurotoxins? Lancet Neurology. 2003;2(9):531–538. doi: 10.1016/s1474-4422(03)00501-5. [DOI] [PubMed] [Google Scholar]
- 59.Marella M., Seo B. B., Yagi T., Matsuno-Yagi A. Parkinson's disease and mitochondrial complex I: a perspective on the Ndi1 therapy. Journal of Bioenergetics and Biomembranes. 2009;41(6):493–497. doi: 10.1007/s10863-009-9249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pryor W. A., Porter N. A. Suggested mechanisms for the production of 4-hydroxy-2-nonenal from the autoxidation of polyunsaturated fatty acids. Free Radical Biology and Medicine. 1990;8(6):541–543. doi: 10.1016/0891-5849(90)90153-A. [DOI] [PubMed] [Google Scholar]
- 61.Pillon N. J., Croze M. L., Vella R. E., Soulère L., Lagarde M., Soulage C. O. The lipid peroxidation by-product 4-hydroxy-2-nonenal (4-HNE) induces insulin resistance in skeletal muscle through both carbonyl and oxidative stress. Endocrinology. 2012;153(5):2099–2111. doi: 10.1210/en.2011-1957. [DOI] [PubMed] [Google Scholar]
- 62.Rahman I., van Schadewijk A. A. M., Crowther A. J. L., et al. 4-Hydroxy-2-nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary disease. The American Journal of Respiratory and Critical Care Medicine. 2002;166(4):490–495. doi: 10.1164/rccm.2110101. [DOI] [PubMed] [Google Scholar]
- 63.Näsström T., Wahlberg T., Karlsson M., et al. The lipid peroxidation metabolite 4-oxo-2-nonenal cross-links α-synuclein causing rapid formation of stable oligomers. Biochemical and Biophysical Research Communications. 2009;378(4):872–876. doi: 10.1016/j.bbrc.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 64.Näsström T., Fagerqvist T., Barbu M., et al. The lipid peroxidation products 4-oxo-2-nonenal and 4-hydroxy-2-nonenal promote the formation of α-synuclein oligomers with distinct biochemical, morphological, and functional properties. Free Radical Biology and Medicine. 2011;50(3):428–437. doi: 10.1016/j.freeradbiomed.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 65.Davidson W. S., Jonas A., Clayton D. F., George J. M. Stabilization of α-synuclein secondary structure upon binding to synthetic membranes. Journal of Biological Chemistry. 1998;273(16):9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 66.Liu S., Ninan I., Antonova I., et al. α-synuclein produces a long-lasting increase in neurotransmitter release. The EMBO Journal. 2004;23(22):4506–4516. doi: 10.1038/sj.emboj.7600451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clayton D. F., George J. M. Synucleins in synaptic plasticity and neurodegenerative disorders. Journal of Neuroscience Research. 1999;58(1):120–129. doi: 10.1002/(sici)1097-4547(19991001)58:160;120::aid-jnr1262;3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 68.Xiang W., Schlachetzki J. C. M., Helling S., et al. Oxidative stress-induced posttranslational modifications of alpha-synuclein: specific modification of alpha-synuclein by 4-hydroxy-2-nonenal increases dopaminergic toxicity. Molecular and Cellular Neuroscience. 2013;54:71–83. doi: 10.1016/j.mcn.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 69.Olanow C. W., Brundin P. Parkinson's disease and alpha synuclein: is Parkinson's disease a prion-like disorder? Movement Disorders. 2013;28(1):31–40. doi: 10.1002/mds.25373. [DOI] [PubMed] [Google Scholar]
- 70.Schinella G., Mosca S., Cienfuegos-Jovellanos E., et al. Antioxidant properties of polyphenol-rich cocoa products industrially processed. Food Research International. 2010;43(6):1614–1623. doi: 10.1016/j.foodres.2010.04.032. [DOI] [Google Scholar]
- 71.Othman A., Ismail A., Abdul Ghani N., Adenan I. Antioxidant capacity and phenolic content of cocoa beans. Food Chemistry. 2007;100(4):1523–1530. doi: 10.1016/j.foodchem.2005.12.021. [DOI] [Google Scholar]
- 72.Lamuela-Raventós R. M., Romero-Pérez A. I., Andrés-Lacueva C., Tornero A. Review: health effects of cocoa flavonoids. Food Science and Technology International. 2005;11(3):159–176. doi: 10.1177/1082013205054498. [DOI] [Google Scholar]
- 73.Dawson T. M., Dawson V. L. Neuroprotective and neurorestorative strategies for Parkinson's disease. Nature Neuroscience. 2002;5:S1058–S1061. doi: 10.1038/nn941. [DOI] [PubMed] [Google Scholar]
- 74.Frei B., Higdon J. V. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. Journal of Nutrition. 2003;133(10):S3275–S3284. doi: 10.1093/jn/133.10.3275S. [DOI] [PubMed] [Google Scholar]
- 75.Chaturvedi R. K., Shukla S., Seth K., et al. Neuroprotective and neurorescue effect of black tea extract in 6-hydroxydopamine-lesioned rat model of Parkinson's disease. Neurobiology of Disease. 2006;22(2):421–434. doi: 10.1016/j.nbd.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 76.Chen C., Yu R., Owuor E. D., Tony Kong A.-N. Activation of antioxidant-response element (ARE), mitogen-activated protein kinases (MAPKs) and caspases by major green tea polyphenol components during cell survival and death. Archives of Pharmacal Research. 2000;23(6):605–612. doi: 10.1007/bf02975249. [DOI] [PubMed] [Google Scholar]
- 77.Imamura K., Hishikawa N., Sawada M., Nagatsu T., Yoshida M., Hashizume Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson's disease brains. Acta Neuropathologica. 2003;106(6):518–526. doi: 10.1007/s00401-003-0766-2. [DOI] [PubMed] [Google Scholar]
- 78.Beach T. G., Sue L. I., Walker D. G., et al. Marked microglial reaction in normal aging human substantia nigra: correlation with extraneuronal neuromelanin pigment deposits. Acta Neuropathologica. 2007;114(4):419–424. doi: 10.1007/s00401-007-0250-5. [DOI] [PubMed] [Google Scholar]
- 79.Sawada M., Imamura K., Nagatsu T. Role of cytokines in inflammatory process in Parkinson's disease. Journal of Neural Transmission. 2006;(70):373–381. doi: 10.1007/978-3-211-45295-0_57. [DOI] [PubMed] [Google Scholar]
- 80.Banati R. B., Daniel S. E., Blunt S. B. Glial pathology but absence of apoptotic nigral neurons in long-standing Parkinson's disease. Movement Disorders. 1998;13(2):221–227. doi: 10.1002/mds.870130205. [DOI] [PubMed] [Google Scholar]
- 81.Jack C. S., Arbour N., Manusow J., et al. TLR signaling tailors innate immune responses in human microglia and astrocytes. The Journal of Immunology. 2005;175(7):4320–4330. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- 82.Largo R., Alvarez-Soria M. A., Díez-Ortego I., et al. Glucosamine inhibits IL-1β-induced NFκB activation in human osteoarthritic chondrocytes. Osteoarthritis and Cartilage. 2003;11(4):290–298. doi: 10.1016/s1063-4584(03)00028-1. [DOI] [PubMed] [Google Scholar]
- 83.Raza S. S., Khan M. M., Ahmad A., et al. Neuroprotective effect of naringenin is mediated through suppression of NF-κB signaling pathway in experimental stroke. Neuroscience. 2013;230:157–171. doi: 10.1016/j.neuroscience.2012.10.041. [DOI] [PubMed] [Google Scholar]
- 84.Kurkowska-Jastrzȩbska I., Babiuch M., Joniec I., Przybyłkowski A., Członkowski A., Członkowska A. Indomethacin protects against neurodegeneration caused by MPTP intoxication in mice. International Immunopharmacology. 2002;2(8):1213–1218. doi: 10.1016/S1567-5769(02)00078-4. [DOI] [PubMed] [Google Scholar]
- 85.Di Matteo V., Pierucci M., Di Giovanni G., et al. Aspirin protects striatal dopaminergic neurons from neurotoxin-induced degeneration: an in vivo microdialysis study. Brain Research. 2006;1095(1):167–177. doi: 10.1016/j.brainres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 86.Johnston L. C., Su X., Maguire-Zeiss K., et al. Human interleukin-10 gene transfer is protective in a rat model of parkinson's disease. Molecular Therapy. 2008;16(8):1392–1399. doi: 10.1038/mt.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Du Y., Ma Z., Lin S., et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(25):14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yoon J.-H., Baek S. J. Molecular targets of dietary polyphenols with anti-inflammatory properties. Yonsei Medical Journal. 2005;46(5):585–596. doi: 10.3349/ymj.2005.46.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jia Z., Babu P. V. A., Si H., et al. Genistein inhibits TNF-α-induced endothelial inflammation through the protein kinase pathway A and improves vascular inflammation in C57BL/6 mice. International Journal of Cardiology. 2013;168(3):2637–2645. doi: 10.1016/j.ijcard.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qureshi A. A., Guan X. Q., Reis J. C., et al. Inhibition of nitric oxide and inflammatory cytokines in LPS-stimulated murine macrophages by resveratrol, a potent proteasome inhibitor. Lipids in Health and Disease. 2012;11, article 76 doi: 10.1186/1476-511x-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen X., Yang X., Liu T., et al. Kaempferol regulates MAPKs and NF-κB signaling pathways to attenuate LPS-induced acute lung injury in mice. International Immunopharmacology. 2012;14(2):209–216. doi: 10.1016/j.intimp.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 92.Zhu X., Zeng K., Qiu Y., Yan F., Lin C. Therapeutic effect of emodin on collagen-induced arthritis in mice. Inflammation. 2013;36(6):1253–1259. doi: 10.1007/s10753-013-9663-6. [DOI] [PubMed] [Google Scholar]
- 93.Choi J. S., Nurul Islam M., Yousof Ali M., Kim E. J., Kim Y. M., Jung H. A. Effects of C-glycosylation on anti-diabetic, anti-Alzheimer's disease and anti-inflammatory potential of apigenin. Food and Chemical Toxicology. 2014;64:27–33. doi: 10.1016/j.fct.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 94.Burlacu A. Regulation of apoptosis by Bcl-2 family proteins. Journal of Cellular and Molecular Medicine. 2003;7(3):249–257. doi: 10.1111/j.1582-4934.2003.tb00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Renault T. T., Teijido O., Antonsson B., Dejean L. M., Manon S. Regulation of Bax mitochondrial localization by Bcl-2 and Bcl-x L: keep your friends close but your enemies closer. The International Journal of Biochemistry & Cell Biology. 2013;45(1):64–67. doi: 10.1016/j.biocel.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 96.Korsmeyer S. J., Shutter J. R., Veis D. J., Merry D. E., Oltvai Z. N. Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Seminars in Cancer Biology. 1993;4(6):327–332. [PubMed] [Google Scholar]
- 97.Gao Q.-G., Xie J.-X., Wong M.-S., Chen W.-F. IGF-I receptor signaling pathway is involved in the neuroprotective effect of genistein in the neuroblastoma SK-N-SH cells. European Journal of Pharmacology. 2012;677(1–3):39–46. doi: 10.1016/j.ejphar.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 98.Chang L., Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410(6824):37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 99.Dérijard B., Raingeaud J., Barrett T., et al. Independent human MAP kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267(5198):682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 100.Whitmarsh A. J., Davis R. J. Role of mitogen-activated protein kinase kinase 4 in cancer. Oncogene. 2007;26(22):3172–3184. doi: 10.1038/sj.onc.1210410. [DOI] [PubMed] [Google Scholar]
- 101.Bhattacharya U., Halder B., Mukhopadhyay S., Giri A. K. Role of oxidation-triggered activation of JNK and p38 MAPK in black tea polyphenols induced apoptotic death of A375 cells. Cancer Science. 2009;100(10):1971–1978. doi: 10.1111/j.1349-7006.2009.01251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cho E. S., Jang Y. J., Kang N. J., et al. Cocoa procyanidins attenuate 4-hydroxynonenal-induced apoptosis of PC12 cells by directly inhibiting mitogen-activated protein kinase kinase 4 activity. Free Radical Biology and Medicine. 2009;46(10):1319–1327. doi: 10.1016/j.freeradbiomed.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 103.Lenaz G. Role of mitochondria in oxidative stress and ageing. Biochimica et Biophysica Acta. 1998;1366(1-2):53–67. doi: 10.1016/s0005-2728(98)00120-0. [DOI] [PubMed] [Google Scholar]
- 104.van Loo G., Saelens X., van Gurp M., MacFarlane M., Martin S. J., Vandenabeele P. The role of mitochondrial factors in apoptosis: a Russian roulette with more than one bullet. Cell Death and Differentiation. 2002;9(10):1031–1042. doi: 10.1038/sj.cdd.4401088. [DOI] [PubMed] [Google Scholar]
- 105.Schapira A. H. V. Mitochondrial dysfunction in Parkinson's disease. Cell Death and Differentiation. 2007;14(7):1261–1266. doi: 10.1038/sj.cdd.4402160. [DOI] [PubMed] [Google Scholar]
- 106.Lev N., Melamed E., Offen D. Apoptosis and Parkinson's disease. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003;27(2):245–250. doi: 10.1016/s0278-5846(03)00019-8. [DOI] [PubMed] [Google Scholar]
- 107.Schapira A. H. V., Cooper J. M., Dexter D., Jenner P., Clark J. B., Marsden C. D. Mitochondrial complex I deficiency in Parkinson's disease. The Lancet. 1989;1(8649):p. 1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- 108.Block K., Gorin Y. Aiding and abetting roles of NOX oxidases in cellular transformation. Nature Reviews Cancer. 2012;12(9):627–637. doi: 10.1038/nrc3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li X., Fang P., Mai J., Choi E. T., Wang H., Yang X.-F. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. Journal of Hematology & Oncology. 2013;6, article 19 doi: 10.1186/1756-8722-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bulua A. C., Simon A., Maddipati R., et al. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) Journal of Experimental Medicine. 2011;208(3):519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nature Reviews Neuroscience. 2003;4(1):49–60. doi: 10.1038/nrn1007. [DOI] [PubMed] [Google Scholar]
- 112.Castellani R. J., Rolston R. K., Smith M. A. Alzheimer disease. Disease-a-Month. 2010;56(9):484–546. doi: 10.1016/j.disamonth.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Camilleri A., Zarb C., Caruana M., et al. Mitochondrial membrane permeabilisation by amyloid aggregates and protection by polyphenols. Biochimica et Biophysica Acta—Biomembranes. 2013;1828(11):2532–2543. doi: 10.1016/j.bbamem.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 114.Büeler H. Impaired mitochondrial dynamics and function in the pathogenesis of Parkinson's disease. Experimental Neurology. 2009;218(2):235–246. doi: 10.1016/j.expneurol.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 115.Zhang M., Swarts S. G., Yin L., et al. Antioxidant properties of quercetin. Advances in Experimental Medicine and Biology. 2011;701:283–289. doi: 10.1007/978-1-4419-7756-4-38. [DOI] [PubMed] [Google Scholar]
- 116.Zheng S. Y., Li Y., Jiang D., Zhao J., Ge J. F. Anticancer effect and apoptosis induction by quercetin in the human lung cancer cell line A-549. Molecular Medicine Reports. 2012;5(3):822–826. doi: 10.3892/mmr.2011.726. [DOI] [PubMed] [Google Scholar]
- 117.Kleemann R., Verschuren L., Morrison M., et al. Anti-inflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis. 2011;218(1):44–52. doi: 10.1016/j.atherosclerosis.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 118.Ganesan S., Faris A. N., Comstock A. T., et al. Quercetin inhibits rhinovirus replication in vitro and in vivo . Antiviral Research. 2012;94(3):258–271. doi: 10.1016/j.antiviral.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Valenti D., De Rasmo D., Signorile A., et al. Epigallocatechin-3-gallate prevents oxidative phosphorylation deficit and promotes mitochondrial biogenesis in human cells from subjects with Down's syndrome. Biochimica et Biophysica Acta—Molecular Basis of Disease. 2013;1832(4):542–552. doi: 10.1016/j.bbadis.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 120.Sonee M., Sum T., Wang C., Mukherjee S. K. The soy isoflavone, genistein, protects human cortical neuronal cells from oxidative stress. NeuroToxicology. 2004;25(5):885–891. doi: 10.1016/j.neuro.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 121.Vallés S. L., Borrás C., Gambini J., et al. Oestradiol or genistein rescues neurons from amyloid beta-induced cell death by inhibiting activation of p38. Aging Cell. 2008;7(1):112–118. doi: 10.1111/j.1474-9726.2007.00356.x. [DOI] [PubMed] [Google Scholar]
- 122.Jagetia G. C., Reddy T. K. Modulation of radiation-induced alteration in the antioxidant status of mice by naringin. Life Sciences. 2005;77(7):780–794. doi: 10.1016/j.lfs.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 123.Jeon S. M., Bok S. H., Jang M. K., et al. Antioxidative activity of naringin and lovastatin in high cholesterol-fed rabbits. Life Sciences. 2001;69(24):2855–2866. doi: 10.1016/s0024-3205(01)01363-7. [DOI] [PubMed] [Google Scholar]
- 124.Jung G., Hennings G., Pfeifer M., Bessler W. G. Interaction of metal-complexing compounds with lymphocytes and lymphoid cell lines. Molecular Pharmacology. 1983;23(3):698–702. [PubMed] [Google Scholar]
- 125.Ryu E. J., Angelastro J. M., Greene L. A. Analysis of gene expression changes in a cellular model of Parkinson disease. Neurobiology of Disease. 2005;18(1):54–74. doi: 10.1016/j.nbd.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 126.Magalingam K. B., Radhakrishnan A., Ramdas P., Haleagrahara N. Quercetin glycosides induced neuroprotection by changes in the gene expression in a cellular model of Parkinson's disease. Journal of Molecular Neuroscience. 2015;55(3):609–617. doi: 10.1007/s12031-014-0400-x. [DOI] [PubMed] [Google Scholar]
- 127.Chen J.-C., Ho F.-M., Chao P.-D. L., et al. Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IκB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. European Journal of Pharmacology. 2005;521(1-3):9–20. doi: 10.1016/j.ejphar.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 128.Xie Q.-W., Whisnant R., Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon γ and bacterial lipopolysaccharide. Journal of Experimental Medicine. 1993;177(6):1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Morse D., Choi A. M. K. Heme oxygenase-1: the ‘emerging molecule’ has arrived. American Journal of Respiratory Cell and Molecular Biology. 2002;27(1):8–16. doi: 10.1165/ajrcmb.27.1.4862. [DOI] [PubMed] [Google Scholar]
- 130.Liu L.-X., Chen W.-F., Xie J.-X., Wong M.-S. Neuroprotective effects of genistein on dopaminergic neurons in the mice model of Parkinson's disease. Neuroscience Research. 2008;60(2):156–161. doi: 10.1016/j.neures.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 131.Zimmermann A. K., Loucks F. A., Schroeder E. K., Bouchard R. J., Tyler K. L., Linseman D. A. Glutathione binding to the Bcl-2 homology-3 domain groove: a molecular basis for Bcl-2 antioxidant function at mitochondria. The Journal of Biological Chemistry. 2007;282(40):29296–29304. doi: 10.1074/jbc.m702853200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Garcia-Segura L. M., Cardona-Gomez P., Naftolin F., Chowen J. A. Estradiol upregulates Bcl-2 expression in adult brain neurons. NeuroReport. 1998;9(4):593–597. doi: 10.1097/00001756-199803090-00006. [DOI] [PubMed] [Google Scholar]
- 133.Dubal D. B., Shughrue P. J., Wilson M. E., Merchenthaler I., Wise P. M. Estradiol modulates bcl-2 in cerebral ischemia: a potential role for estrogen receptors. The Journal of Neuroscience. 1999;19(15):6385–6393. doi: 10.1523/JNEUROSCI.19-15-06385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Singer C. A., Rogers K. L., Dorsa D. M. Modulation of Bcl-2 expression: a potential component of estrogen protection in NT2 neurons. NeuroReport. 1998;9(11):2565–2568. doi: 10.1097/00001756-199808030-00025. [DOI] [PubMed] [Google Scholar]
- 135.Levites Y., Amit T., Youdim M. B. H., Mandel S. Involvement of protein kinase C activation and cell survival/cell cycle genes in green tea polyphenol (−)-epigallocatechin 3-gallate neuroprotective action. The Journal of Biological Chemistry. 2002;277(34):30574–30580. doi: 10.1074/jbc.m202832200. [DOI] [PubMed] [Google Scholar]


