We first reviewed the English-language literature for reported cases of Trichosporon fungemia over the past four decades, and did comprehensive analysis in order to guide our understanding of epidemiology and outcome-related aspects, especially the antifungal treatment and CVC management.
Keywords: epidemiology, prognostic factor, therapeutic strategy, Trichosporon fungemia
Abstract
Background. Trichosporon species have emerged as an important non-Candida spp yeast pathogen in immunocompromised patients in recent decades; however, the systemic analysis of Trichosporon epidemiology has seldom been reported.
Methods. We reviewed 185 reported cases of Trichosporon fungemia from 1975 to 2014 in the English-language literature, and the epidemiology and prognostic factors of the included cases are described.
Results. The number of cases reported has increased with time, especially over the past decade. During the 3 decades from 1975 to 2004, the most commonly used antifungal compounds were amphotericin B/liposomal amphotericin B; however, in recent decades (2005–2014), triazoles (especially voriconazole) have become the most widely used agents, significantly improving outcome in the reported cases. Correlation analysis revealed that negative outcome is associated with several prognostic factors, including a history of antimicrobial use, bacterial bloodstream coinfection, prophylactic/empirical antifungal therapy, Trichosporon beigelii infection, and receiving the antifungal regimen of amphotericin B/liposomal amphotericin B. In addition, a significantly greater proportion of patients with a positive outcome had fungemia without invasive tissue infection and received a voriconazole regimen or an AmB-triazole combined regimen. Significant positive outcome was also associated with patients who had recovered from neutropenia or after central venous catheter removal.
Conclusions. Voriconazole can be recommended as a first-line antifungal compound to treat Trichosporon fungemia; the immune status of the host plays a crucial role in the outcome of this infection, and the removal of vascular catheters should be considered if feasible.
Trichosporon species have emerged as important human pathogens during the past 4 decades in association with an increased number of immunocompromised hosts [1]. Invasive trichosporonosis (IT) is an emerging fatal opportunistic infection and it predominantly occurs in immunocompromised hosts; this infection occurs particularly in patients with hematological malignant diseases (HMDs) and occasionally in those with no apparent immune impairment [2]. Invasive trichosporonosis can involve most organs of the human body, Trichosporon fungemia (TF), including catheter-related fungemia, represents the main type of this opportunistic infection, which accounts for 58.8%–74.7% of infections [2–4]. Although Trichosporon spp currently represent the second most common yeast fungemia in patients with HMDs [5], TF is often neglected and clinically misdiagnosed as other types of yeast fungemia, especially candidemia [6].
Phenotypic and biochemical techniques are routinely used to identify Trichosporon species; however, these techniques are time consuming and are often inaccurate for species identification [7, 8]. Thus, early and accurate diagnosis remains challenging. Although some recommendations and 1 guideline regarding the use of triazoles for the treatment of IT have been published [5, 9, 10], these recommendations are mainly based on low-level evidence obtained from in vitro susceptibility tests, animal models, and case reports/case series rather than from randomized control trials. Information on the use of voriconazole for the treatment of TF and other related clinical issues, such as the management of vascular catheters and neutrophil recovery, is not enough. Thus, selecting the appropriate strategy of managing TF remains difficult.
Due to delayed diagnosis and the lack of an optimal treatment strategy, TF mortality remains high (53%–76%) [3, 11]. Due to the low incidence and difficulty in diagnosis, most reports of TF are limited to individual case descriptions or relatively small case series involving single institutions, and no comprehensive analysis has been reported to guide our understanding of the epidemiology, therapeutic aspects, and outcome of this infection; moreover, the prognostic factors associated with outcomes remained unclear [11]. Therefore, to improve our knowledge of this uncommon infection, we reviewed the English-language literature for cases of TF that have been reported over the past 4 decades, including original case reports and case series, to elucidate the epidemiology and prognostic factors associated with patient outcome.
MATERIALS AND METHODS
To ensure accuracy, 2 independent reviewers (Y. L. and S. Y.) separately conducted a literature review using PubMed (http://www.ncbi.nlm.nih.gov/pubmed) to identify all English-language articles mentioning TF for the period from 1975 to 2014. Search keywords included “Trichosporon”, “trichosporonosis”, “Trichosporon fungemia”, “trichosporonomia”, and “Trichosporon bloodstream infection”. After reviewing the recovered articles, references cited in the articles were reviewed to identify additional case reports and case series. Cases were included if at least 1 Trichosporon spp was isolated in a blood culture and the clinical syndrome was consistent with infection. Cases of superficial infection and infection with Trichosporon capitatum or Trichosporon pullulans were excluded because these species have been reclassified to a different genus. The reports that were reviewed in further analyses are listed in the Supplementary Materials. For each case, the following data were collected (if available): publication year, geographic location, basic demographics and clinical characteristics, underlying conditions, microbiology, treatment, and outcome. We gave special attention to therapeutic modalities, such as antifungal treatments and catheter removal. To test the trends over time, we grouped the TF into four 10-year periods. We listed the number of TF cases reported in each of these 4 periods and categorized the results according to geographic location, species, antifungal treatment, and outcome. Cases that were reported in detail were included in a correlation analysis of potential prognostic factors (among the variables collected) and outcome by performing a χ2 test or a Fisher exact test for categorical variables. A P value of <.05 was considered statistically significant, and a P value of <.0083 was considered statistically significant for multiple comparisons of the outcome of 4 treatment regimens. All statistical calculations were performed using standard programs implemented in SAS version 9.3.
RESULTS
The literature review yielded 185 cases of TF from 1975 to 2014, and the number of reported cases generally increased during this time (Figure 1). Cases were reported in 4 continents and had been changed from mainly in North America and Europe in the early stage (1975–2004) to in Asia and South America in the late stage (2005–2014) (Figure 1). The mean age of the 184 infected patients was 47 years (ranging from 0 to 84). Most cases were male (121 of 183, 66.12%), and the male/female ratio was 1.95:1. Hematological diseases (106 of 185, 57.30%) (with acute leukemia 82 of 106, 77.36% in HMDs), premature birth (14 of 185, 7.57%), diabetes (9 of 185, 4.86%), and solid tumor (8 of 185, 4.32%) were the 4 most common underlying diseases or conditions. Most patients had a history of neutropenia (60.49%), chemotherapy treatment (58.44%), antimicrobial use (84.05%), prophylactic/empirical antifungal therapy (43.24%), or central venous catheter (CVC) use (52.81%) before or at the onset (present on admission [POA]) of TF, and some patients had a history of corticosteroids use (22.70%), intensive care unit admission (25.32%), or bacterial bloodstream coinfection (25%). A total of 110 TF cases (59.46%) involved single invasive tissue infection or disseminated infections. Together with nonspecific and systemic symptoms (eg, fever and asthenia), local symptoms related to a specific site of infection were also reported.
Figure 1.
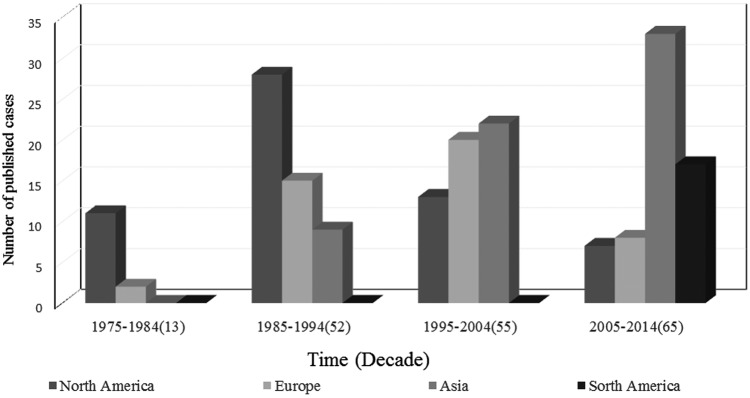
Geographic distribution of 185 patients with Trichosporon fungemia over 4 decades (1975–2014).
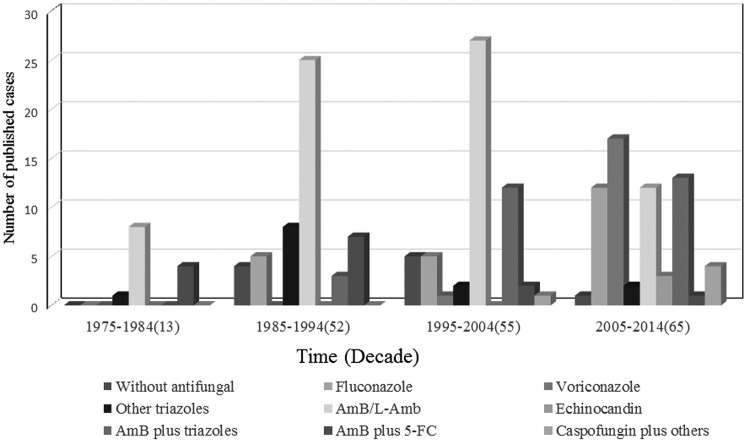
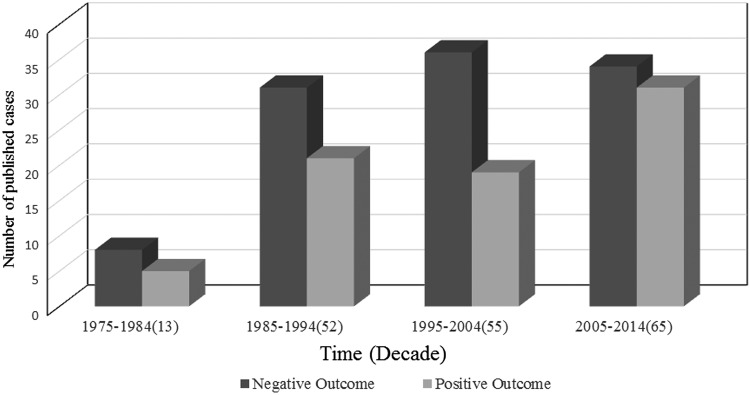
A total of 185 Trichosporon spp strains were isolated from blood samples; a total of 76 isolates were classified at the species level according to the revised classification [12, 13], which mainly reported after 2005. The identification methods used were described for major isolates (131 of 185, 70.81%) but not for minor isolates (54 of 185, 29.19%). Several methods have been used singly or in combination to identify Trichosporon species in these reports, including morphological methods (5 of 185, 2.7%), biochemical tests (91 of 185, 49.19%) (API 20C AUX, ID 32C, the Microscan Rapid Yeast ID system, the Uni-Yeast-Tek system, and Vitek Systems), and molecular methods (35 of 185, 18.92%) (nucleotide sequences of SSU, LSU D1/D2, ITS, and IGS1 regions); the most commonly used method in these cases combined the morphological method with a biochemical test (84 of 45, 41%), and more molecular methods were used in the resent 10-year period (Figure 2). The most commonly isolated Trichosporon species was Trichosporon asahii (57 of 76, 75%); less frequently isolated species included Trichosporon mucoides (5 of 76, 6.58%), Trichosporon inkin (4 of 76, 5.26%), Trichosporon asteroides (4 of 76, 5.26%), Trichosporon loubieri (2 of 76, 2.63%), and 1 case each (1.31%) of Trichosporon cutaneum, Trichosporon dermatis, Trichosporon mycotoxinivorans, and Trichosporon coremiiforme. A total of 109 patients (58.92%) experienced a negative outcome (death/worsening), and 76 patients (41.08%) experienced a positive outcome (cure/improvement). Among the reported cases during the most recent period (2005–2014), 34 patients (52.31%) experienced a negative outcome; this value represents a decrease compared with the other 3 periods (36 patients [65.45%] during 1975–1984, 31 patients [59.62%] during 1985–1994, and 8 patients [61.36%] during 1995–2004; Figure 3).
Figure 2.
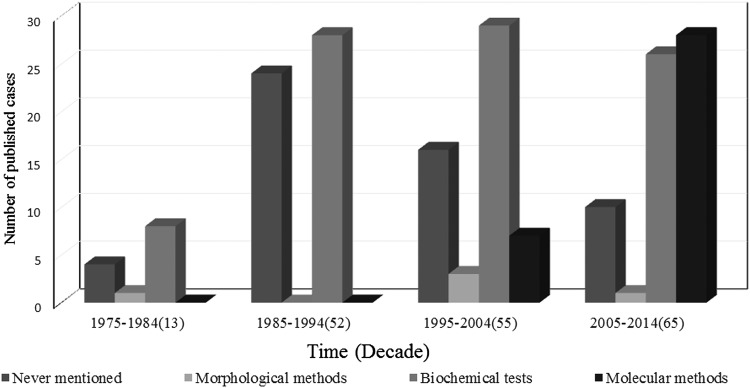
Identification methods distribution of 185 isolates from Trichosporon fungemia over 4 decades (1975–2014).
Figure 3.
Clinical outcome of 185 patients with Trichosporon fungemia over 4 decades (1975–2014).
Representing the 40 years from 1975 to 2014, the 185 cases of TF reviewed in our study were summarized; correlations were assessed between various prognostic factors and clinical outcome. In a univariate analysis, a significantly greater proportion of patients with a negative outcome had a history of using antimicrobials (P = .017), prophylactic/empirical antifungal therapy (P = .015), or bacterial bloodstream coinfection (P = .005) POA of TF (P = .018) (Table 1). In addition, a significantly greater proportion of patients with a positive outcome had fungemia without invasive tissue infection than with invasive tissue infection (P = .012) (Table 1). Likewise, for other variables, no statistically significant differences were found (Table 1). Among 75 patients who had a history of neutropenia POA of TF and for whom the state of neutropenia could be available after the development of TF, 34 exhibited neutrophil recovery. Among 77 patients who had a history of CVC usage POA of TF and for whom the state of CVC could be available after the development of TF, 53 had undergone catheter removal. Among the patients experiencing a positive outcome, a significantly greater proportion followed neutrophil recovery (P < .001) or catheter removal (P = .033) (Table 2).
Table 1.
Distribution of Patient Characteristics Before or at Onset of Trichosporon Fungemia According to Outcome
| Characteristics, no. (%) | Total (n = 185) | Positive (n = 109) | Negative (n = 76) | P Value |
|---|---|---|---|---|
| Age | 184 | 75 | 109 | .114 |
| Infant | 20 | 12 (16) | 8 (7.3) | .063 |
| Child | 26 | 11 (14.7) | 15 (13.8) | .862 |
| Adult | 107 | 44 (58.7) | 63 (57.8) | .906 |
| Elderly | 31 | 8 (10.7) | 23 (21.1) | .063 |
| Gender | 183 | 74 | 109 | .767 |
| Male | 121 | 48 (64.9) | 73 (67.0) | |
| HDs as UDa | 185 | 76 | 109 | .284 |
| Yes | 106 | 40 (52.6) | 66 (60.6) | |
| Chemotherapy | 154 | 66 | 88 | .395 |
| Yes | 90 | 36 (54.5) | 54 (61.4) | |
| Corticosteroids | 163 | 66 | 97 | .129 |
| Yes | 37 | 11 (16.7) | 26 (26.8) | |
| Neutropenia | 162 | 65 | 97 | .156 |
| Yes | 98 | 35 (53.8) | 63 (64.9) | |
| Intensive care unit | 154 | 66 | 88 | .914 |
| Yes | 39 | 17 (25.8) | 22 (25) | |
| Central venous catheter | 178 | 74 | 104 | .981 |
| Yes | 94 | 39 (52.7) | 55 (52.9) | |
| BBCb | 168 | 75 | 93 | .005 |
| Yes | 42 | 11 (14.7) | 31 (33.3) | |
| Use of antimicrobials | 163 | 66 | 97 | .017 |
| Yes | 137 | 50 (75.8) | 87 (89.7) | |
| P/E-ATc | 169 | 67 | 102 | .015 |
| Yes | 80 | 24 (35.8) | 56 (54.9) | |
| Species | 185 | 76 | 109 | .048 |
| TB | 109 | 37 (48.7) | 72 (66.1) | .018 |
| Trichosporon asahii | 57 | 28 (36.8) | 29 (26.6) | .137 |
| Non-asahii Trichosporond | 19 | 11 (14.5) | 8 (7.3) | .115 |
| Involving S/D infectione | 185 | 76 | 109 | .012 |
| Yes | 110 | 37 (48.7) | 73 (67.0) | |
| Symptoms | 155 | 65 | 90 | .924 |
| Fever only | 77 | 32 (49.2) | 45 (50) | |
| Fever with other symptomsf | 78 | 33 (50.8) | 45 (50) |
Abbreviations: BBC, bacterial bloodstream coinfections; HDs as UD, hematological diseases as underlying diseases; P/E-AT, prophylactic/empirical antifungal therapy; S/D, involving single invasive tissue infection or disseminated infections; TB, identified as Trichosporon beigelii or at the genus level of Trichosporon.
a Underlying hematological disease, including acute/chronic myeloid leukemia, acute/chronic lymphoid leukemia, megaloblastic anemia, lymphoma, aplastic anemia, or other hematological disease.
b Bacterial bloodstream coinfections including Staphylococcus spp, Bacteremia spp, Klebsiella spp, Escherichia coli, Pseudomonas aeruginosa, Enterobacter cloacae, Serratia marcescens, and Streptococcus intermedius.
c Prophylactic/empirical antifungal therapy including amphotericin B (38/80), echinocandins (22/80), fluconazole (10/80), itraconazole (5/80), miconazole (2/80), ketoconazole (1/80), posaconazole (1/80), and voriconazole (1/80).
d Non-asahii Trichosporons including Trichosporon mucoides, Trichosporon inkin, Trichosporon asteroides, Trichosporon loubieri, Trichosporon cutaneum, Trichosporon dermatis, Trichosporon mycotoxinivorans, and Trichosporon coremiiforme.
e Involving single invasive tissue infection or disseminated infections including lung, subskin/soft tissue, liver, kidney, and spleen.
f Fever with other symptoms including skin/mouth lesions, hemorrhage, cough, dyspnoea, hematuria, abdominal pain, chest pain, joint pain, and headache.
Table 2.
Distribution of Patient Characteristics After Confirmed Infection of Trichosporon Fungemia According to the Outcome
| Characteristics, No. (%) | Total (n = 185) | Positive (n = 109) | Negative (n = 76) | P Value |
|---|---|---|---|---|
| Neutrophil recovery | 75 | 30 | 45 | <.001 |
| Yes | 34 | 29 (96.7) | 5 (11.1) | |
| Catheter removal | 77 | 33 | 44 | .033 |
| Yes | 53 | 27 (81.8) | 26 (59.1) | |
| Antifungals | 185 | 76 | 109 | .006 |
| Noa | 10 | 1 (1.3) | 9 (8.3) | .048 |
| Fluconazole | 22 | 12 (15.8) | 10 (9.2) | .171 |
| Voriconazole | 18 | 12 (15.8) | 6 (5.5) | .020 |
| Other azolesb | 13 | 6 (7.9) | 7 (6.4) | .699 |
| AmB | 72 | 21 (27.3) | 51 (46.8) | .008 |
| Echinocandinsc | 3 | 0 (0) | 3 (2.8) | .269 |
| AmB + azolesb | 28 | 17 (22.4) | 11 (10.1) | .021 |
| AmB + 5-FC | 14 | 5 (6.6) | 9 (8.3) | .671 |
| AmB + echinocandinsc | 5 | 2 (2.6) | 3 (2.8) | .960 |
Abbreviations: AmB, amphotericin B/liposomal amphotericin B; 5-FC, 5-fluorocytosine.
a No, without any antifungal.
b Azoles including itraconazole, miconazole, ketoconazole, and posaconazole.
c Echinocandins including caspofungin and micafungin.
Various treatment regimens were used; however, amphotericin B/liposomal amphotericin B (AmB) were the most frequently used monotherapies during the first 3 decades (61.54% during 1975–1984, 48.08% during 1985–1994, and 49.09% during 1995–2004). The therapy with a single triazole has increased to become the most frequently used drug (47.69%) during 2005–2014, and these triazoles include voriconazole (26.15%), fluconazole (18.46%), posaconazole (1.54%), and miconazole (1.54%) (Figure 4). A significantly greater proportion of patients experiencing a negative outcome received the antifungal regimen using AmB (P = .017) (Table 2). However, a significantly greater proportion of patients experiencing a positive outcome received an antifungal regimen using voriconazole (P = .020) or a regimen using an AmB-triazole combination (P = .021). Among the 4 commonly used antifungal compounds, significant differences in positive outcome rate were found between regimens using AmB and voriconazole (P = .003), between AmB and fluconazole (P = .029), and between AmB and the combination of AmB-triazole (P = .004); differences in outcome between voriconazole and fluconazole (P = .436) or between fluconazole and AmB-triazole combination (P = .683) were not statistically significant (Table 3).
Figure 4.
Antifungals administered to 185 patients with Trichosporon fungemia over 4 decades (1975–2014).
Table 3.
Primary Treatment of Patients with Trichosporon Fungemia According to Outcome
| Treatment, No. (%) | Total | Positive | Negative | P Value |
|---|---|---|---|---|
| Fluconazole | 22 | 12 (54.5) | 10 (45.5) | .436 |
| Voriconazole | 18 | 12 (66.7) | 6 (33.3) | |
| Fluconazole | 22 | 12 (54.5) | 10 (45.5) | .029 |
| AmB | 72 | 21 (29.2) | 51 (70.8) | |
| Fluconazole | 22 | 12 (54.5) | 10 (45.5) | .661 |
| AmB + azoles | 28 | 17 (60.7) | 11 (39.3) | |
| Voriconazole | 18 | 12 (66.7) | 6 (33.3) | .003 |
| AmB | 72 | 21 (29.2) | 51 (70.8) | |
| Voriconazole | 18 | 12 (66.7) | 6 (33.3) | .683 |
| AmB + azoles | 28 | 17 (60.7) | 11 (39.3) | |
| AmB | 72 | 21 (29.2) | 51 (70.8) | .003 |
| AmB + azoles | 28 | 17 (60.7) | 11 (39.3) |
Abbreviations: AmB, amphotericin B/liposomal amphotericin B.
DISCUSSION
In modern medicine, many factors are considered to contribute to immunosuppression and physical barriers impairment of hospitalized patients; these factors also contribute to the observed increase in the number of patients with invasive fungal infections, especially for HMDs patients. Fungemia has been recognized with increasing frequency in HMDs patients in recent decades [5, 6, 10]. Trichosporon has emerged as the second most common yeast genus causing fungemia in HMDs patients and is frequently the pathogen of breakthrough infection in patients receiving prophylactic/empirical antifungal therapy [1, 5, 11]. We reviewed 185 cases of TF in English-language publications from the first case reported in 1975. The number of reported cases increased significantly from 13 in the first decade (1975–1984) to 65 in the last decade (2005–2014), consistent with previous research that the incidence of TF has increased in HMDs patients during the period from 1988 to1997 in a national cancer institution [14]. The reason for the changing trend of geographic distribution (the cases increased in South America and Asia) at a late stage is probably attributed to multiple factors, including an increasing concern of doctors and researchers from South America and Asia on Trichosporon infections and improved clinical diagnostic technique for medical mycology in these areas [1, 15]. The real epidemiological trend for IT will be evaluated when more research teams join this field and establish a specific study group or network on Trichosporon infection.
For patients with TF in our study, the most common underlying conditions were HMDs, especially acute leukemia, a finding that is consistent with the results of another study (82% of all HMDs) [11]. In addition, most TF cases were associated with neutropenia (60.49%), chemotherapy (58.44%), antimicrobial use (84.05%), prophylactic/empirical antifungal therapy (47.34%), or CVC (52.81%) use POA of infection. These risk factors were also found to be associated with TF or IT in other studies involving small samples and included neutropenia (85%–91%) [11, 14], chemotherapy (91%) [11, 14], broad-spectrum antimicrobial use (91%) [14], prophylactic/empirical antifungal therapy (58%–91%) [11, 14], or CVC use (42%–100%) [11, 14, 16]. Trichosporon fungemia was more strongly correlated with HMDs and neutropenia than other rare opportunistic (non-Candida, non-Cryptococcus) yeast bloodstream infections (ROYBSIs) [6].
The timing and sensitivity of diagnosis remain important for the management of TF. Based on a molecular analysis, the taxonomy of the genus Trichosporon has been extensively revised; Trichosporon beigelli was replaced by 6 species of Trichosporon as potential human pathogens [17–19]. The genus of Trichosporon was extended to 50 species by 2011 [1]. According to the revised classification, T asahii is found herein to be the most frequently reported clinical isolate (75%) that causes TF, which is similar to that observed previously [2, 16]. The traditional culture and identification of Trichosporon spp from blood samples—in particular, identification to the species level—can be difficult using clinical methods that are routinely used in general microbiology laboratories; moreover, biochemical identification are among the methods that are more often used for the diagnosis of TF, especially in the past 10 years. However, the biochemical database for identifying Trichosporon spp has been poor. Although potentially useful, modern molecular techniques that are based on the sequencing of Trichosporon spp ribosomal DNA cannot be routinely adopted at this time—although they already been used in South America and Asia—representing a challenge for early diagnosis and leading to delays in selecting appropriate antifungal therapies [1, 20]. In our study, 58.92% of patients experienced a negative outcome, and the outcome of TF patients improved slightly over time (Figure 3). However, previous studies reported a higher mortality of TF (from 76% to 83.3%) [11, 14], even after antifungal therapy; this finding might be due to a reporting bias such that more cases with favorable outcomes were reported in our review study.
Patients at high risk of developing TF, especially HMDs patients during the neutropenic phase, are vulnerable to systemic bacterial infections and frequently receive antimicrobial therapy. We found that antimicrobial use POA of TF (which can result in an imbalance of the microbiota) and concomitant bacteremia (which would aggravate the patient's condition) were both associated with a poor prognosis of TF, and a similar result was found in candidemia [21, 22]. When fever or other signs of infection continue despite antimicrobial therapy, patients were frequently considered as having possible invasive fungal infections, rendering prophylactic/empirical treatment necessary. When prophylactic/empirical treatment was used for the neutropenic patients with potential invasive fungal infection, echinocandins or a lipid formulation of amphotericin B were recommended [23], which increased the occurrence of breakthrough infection of Trichosporon spp. Based on analysis of published TF cases, 43.24% (80 of 185) of patients presented with breakthrough TF, mainly related to the use of amphotericin B (38 of 80) and echinocandin (22 of 80). These findings have also been described by others [3, 11, 24–27] and is consistent with the resistant pattern of Trichosporon spp (intrinsic resistance to echinocandins and decreased susceptibility to amphotericin B), which might play a role in the competitive inhibition of selective pressure for the growth of resistant fungi. In case the clinical features of TF cannot be distinguished from candidemia [28], TF treatment has often involved initial therapy with antifungals, to which Trichosporon spp is less sensitive [29]. These reasons might explain why prophylactic/empirical antifungal treatment was related to negative breakthrough TF outcome, which also stresses the importance of early diagnosis.
Central venous catheter use was also found to be one of the most frequent risk factors for TF (52.81%) in our study, consistent with previous studies (41%–100%) [3, 4, 11, 14]. It has been reported that catheters might be a portal of entry for Trichosporon spp [14, 30], and some TFs represented catheter-related bloodstream infections (CR-BSIs) [1, 3, 5, 11]. The formation of Trichosporon biofilms on catheter surfaces, especially CVCs, might play an important role in the pathogenesis of Trichosporon CR-BSIs, diseases that are often difficult to treat and eradicate and that ultimately lead to persistent or recurrent infections despite treatment with antifungals, to which Trichosporon spp is sensitive [1, 31, 32]. Our study revealed that catheter removal was significantly associated with a positive outcome (P = .033) in patients who had a history of CVC use POA of TF. Several studies have demonstrated that catheter removal is also associated with improved survival in patients with candidemia or ROYBSIs [6, 22, 33–35], particularly for the early CVC removal [36, 37]. However, other studies failed to prove the significance of catheter removal in patients with candidemia [38–40]. Although controversy remains regarding neutropenic patients, catheter removal is recommended as an adjunct strategy for treating patients with candidemia [23]. According to our results and the guidelines of candidemia, catheter removal should also be suggested for TF when feasible, and our previous study reveals that ethanol might be a useful choice for preventing and treating T asahii biofilm-related CR-BSIs [41].
The use of an appropriate antifungal treatment is associated with survival in patients with candidemia [21, 22, 34], especially for secondary noncatheter-related candidemia, and early and adequate antifungal therapy (but not catheter withdrawal) was a protective factor for the mortality [34]. Due to the rarity of IT and the lack of randomized control trials, the optimal therapy for IT has not been established. Amphotericin B was first recommended as an initial therapy for IT in 1995 [42]. For TF, we found that AmB (72 of 185, 38.92%) and AmB-triazole (28 of 185, 15.14%) were the most commonly used single-drug treatment and combination treatment, respectively (Table 2); a similar finding (79.6%) has also been reported in a previous literature review in 2005 [2]. Walsh et al [43] reported that 77% of Trichosporon isolates were not killed at achievable amphotericin B serum levels; the finding was correlated with refractory, disseminated trichosporonosis in granulocytopenic patients. The similar resistance profiles of Trichosporon isolates against amphotericin B have been found in previous studies in vitro [16, 44]. We found that a significantly higher proportion of patients receiving AmB experienced a negative prognosis. In patients with IT, poor responses to amphotericin B have been reported [1, 2, 14, 43, 45]. Therefore, amphotericin B should not be recommended for treating Trichosporon infection.
Based on previous experimental and clinical evidence [4, 46–48] from 2005, several reviews and 1 guideline have recommended the use of triazoles for treating IT [2, 5, 9, 10, 49]. We found that azole-included therapy was the most frequently used antifungal treatment for TF (65.7%) after 2004 (Figure 4), which was also reported for IT in another case series [4]. A 16-year retrospective study of TF patients with HMDs found that the resolution of TF was associated with triazole-included therapy [11]. Although fluconazole has previously been recommended for IT, we did not find a significant association between positive outcome and fluconazole treatment; however, we did find that a significantly higher proportion of patients receiving voriconazole experienced positive outcomes. The first successful treatment of IT with voriconazole was reported in 2002 [50], a finding that has been confirmed in other clinical and animal studies [4, 51–53]. Voriconazole has recently been found more effective (in vitro) than amphotericin B, fluconazole, and ravuconazole against most clinical Trichosporon species [16, 54], even for isolates that are resistant to fluconazole, itraconazole, or amphotericin B [55]. Recent guidelines suggest that voriconazole is the preferred agent for treating Trichosporon infections [9]. Consequently, our findings suggest a promising role for voriconazole in the treatment of TF. We also found a significant association between positive outcome and treatment with an AmB-triazole combination. In vitro synergistic effects of antifungal combinations including voriconazole, amphotericin B, and caspofungin against T asahii have been reported [31, 56], and these were confirmed by an animal model experiment [46], but the clinical effectiveness of AmB-triazole combination therapy requires more studies to be confirmed.
It has been recognized that prolonged neutropenia compromises the host defense mechanism against fungal infections [57]. In this study, neutrophil recovery was found to be the main factor affecting outcome in neutropenic patients with TF, which has also been reported in another case series that the resolution of TF was associated with neutrophil recovery for 28 HMDs patients [11]. Other case reports and case series suggest that the improvement of neutropenia is a prognostic factor for patients with IT [58–60]. Despite receiving antifungal therapy, IT in patients with persistent profound neutropenia might fail to respond to treatment with triazoles [48], a finding that emphasizes the importance of the patient's immune status in determining the outcome of Trichosporon infections [61]. Neutropenia recovery has been reported as an independent factor for candidemia survival in patients with HMDs [62]. To reduce the duration of neutropenia, immunosuppressive therapy should be reduced or terminated if clinically feasible, and the administration of granulocyte macrophage colony-stimulating factor (CSF) or granulocyte CSF can be considered [63], which has been shown to enhance the fungicidal activity of human monocytes against Trichosporon species in vitro [64], and have been used in neutropenic patients with Candida infection to assist bone marrow recovery after the remission of neutropenia [65].
Our study has several limitations. First, this was a retrospective study; therefore, some information might not have been well documented in the patients' charts, including illness severity, antifungal dose and duration, and the timing of catheter removal. Second, as a retrospective, multisource case review study, the possibility of a reporting bias cannot be eliminated because cases with more favorable outcomes were reported. Finally, it would have been better to use a multivariate analysis to explore the prognostic factors for clinical outcome. However, when running a logistic regression, quasi-complete separation can be problematic. Quasi-complete separation occurs when studying many categorical variables and using a small sample size. Therefore, the results should be interpreted with extreme caution, and further development of national databases and well defined multicenter studies are needed to resolve these limitations and confirm our results.
CONCLUSIONS
In summary, this study represents the largest review of TF published to date, and it provides useful information about this uncommon infection by describing its epidemiology, treatment, and outcome. Trichosporon fungemia is frequently difficult to diagnose, refractory to treatment, and associated with high mortality. Our results emphasize the fact that TF has played a more important role in the most recent decade, especially in HMDs patients treated with broad-spectrum antimicrobials and in patients with indwelling devices during neutropenia. Many common antifungals have limitations in the treatment of TF; however, triazoles, especially voriconazole, are expected to improve patient outcome and play a key role in future antifungal strategies. Patients with neutropenia usually do not recover from infection despite antifungal therapy, unless the neutropenia recovery; however, the removal of catheters is associated with positive survival.
Supplementary Data
Supplementary material is available online at Open Forum Infectious Diseases online (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
Financial support. This work was supported by the National Natural Science Foundation of China (grant nos. 81301410 and 81471928).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Colombo AL, Padovan AC, Chaves GM. Current knowledge of Trichosporon spp. and trichosporonosis. Clin Microbiol Rev 2011; 24:682–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Girmenia C, Pagano L, Martino B et al. . Invasive infections caused by Trichosporon species and Geotrichum capitatum in patients with hematological malignancies: a retrospective multicenter study from Italy and review of the literature. J Clin Microbiol 2005; 43:1818–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kontoyiannis DP, Torres HA, Chagua M et al. . Trichosporonosis in a tertiary care cancer center: risk factors, changing spectrum and determinants of outcome. Scand J Infect Dis 2004; 36:564–9. [DOI] [PubMed] [Google Scholar]
- 4.Ruan SY, Chien JY, Hsueh PR. Invasive trichosporonosis caused by Trichosporon asahii and other unusual Trichosporon species at a medical center in Taiwan. Clin Infect Dis 2009; 49:e11–7. [DOI] [PubMed] [Google Scholar]
- 5.Miceli MH, Diaz JA, Lee SA. Emerging opportunistic yeast infections. Lancet Infect Dis 2011; 11:142–51. [DOI] [PubMed] [Google Scholar]
- 6.Chitasombat MN, Kofteridis DP, Jiang Y et al. . Rare opportunistic (non-Candida, non-Cryptococcus) yeast bloodstream infections in patients with cancer. J Infect 2012; 64:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmad S, Al-Mahmeed M, Khan ZU. Characterization of Trichosporon species isolated from clinical specimens in Kuwait. J Med Microbiol 2005; 54:639–46. [DOI] [PubMed] [Google Scholar]
- 8.Taj-Aldeen SJ, Al-Ansari N, El Shafei S et al. . Molecular identification and susceptibility of Trichosporon species isolated from clinical specimens in Qatar: isolation of Trichosporon dohaense Taj-Aldeen, Meis & Boekhout sp. nov. J Clin Microbiol 2009; 47:1791–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arendrup MC, Boekhout T, Akova M et al. . ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin Microbiol Infect 2014; 20:76–98. [DOI] [PubMed] [Google Scholar]
- 10.Caira M, Trecarichi EM, Tumbarello M et al. . Uncommon yeast infections in hematological patients: from diagnosis to treatment. Expert Rev Anti Infect Ther 2011; 9:1067–75. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki K, Nakase K, Kyo T et al. . Fatal Trichosporon fungemia in patients with hematologic malignancies. Eur J Haematol 2010; 84:441–7. [DOI] [PubMed] [Google Scholar]
- 12.Gueho E, de Hoog GS, Smith MT. Neotypification of the genus Trichosporon. Antonie Van Leeuwenhoek 1992; 61:285–8. [DOI] [PubMed] [Google Scholar]
- 13.Gueho E, Smith MT, de Hoog GS et al. . Contributions to a revision of the genus Trichosporon. Antonie van Leeuwenhoek 1992; 61:289–316. [DOI] [PubMed] [Google Scholar]
- 14.Krcmery V Jr, Mateicka F, Kunova A et al. . Hematogenous trichosporonosis in cancer patients: report of 12 cases including 5 during prophylaxis with itraconazol. Support Care Cancer 1999; 7:39–43. [DOI] [PubMed] [Google Scholar]
- 15.Marine M, Brown NA, Riano-Pachon DM et al. . On and under the skin: emerging basidiomycetous yeast infections caused by Trichosporon species. PLoS Pathog 2015; 11:e1004982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chagas-Neto TC, Chaves GM, Melo AS et al. . Bloodstream infections due to Trichosporon spp. species distribution, Trichosporon asahii genotypes determined on the basis of ribosomal DNA intergenic spacer 1 sequencing, and antifungal susceptibility testing. J Clin Microbiol 2009; 47:1074–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugita T, Nishikawa A, Shinoda T et al. . Taxonomic position of deep-seated, mucosa-associated, and superficial isolates of Trichosporon cutaneum from trichosporonosis patients. J Clin Microbiol 1995; 33:1368–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugita T, Nishikawa A, Ikeda R et al. . Identification of medically relevant Trichosporon species based on sequences of internal transcribed spacer regions and construction of a database for Trichosporon identification. J Clin Microbiol 1999; 37:1985–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gueho E, Improvisi L, de Hoog GS et al. . Trichosporon on humans: a practical account. Mycoses 1994; 37:3–10. [DOI] [PubMed] [Google Scholar]
- 20.Zhou J, Liao Y, Li H et al. . Development of a loop-mediated isothermal amplification assay for rapid detection of Trichosporon asahii in experimental and clinical samples. BioMed Res Int 2015; 2015:732573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boo TW, O'Reilly B, O'Leary J et al. . Candidaemia in an Irish tertiary referral hospital: epidemiology and prognostic factors. Mycoses 2005; 48:251–9. [DOI] [PubMed] [Google Scholar]
- 22.Alonso-Valle H, Acha O, Garcia-Palomo JD et al. . Candidemia in a tertiary care hospital: epidemiology and factors influencing mortality. Eur J Clin Microbiol Infect Dis 2003; 22:254–7. [DOI] [PubMed] [Google Scholar]
- 23.Pappas PG, Kauffman CA, Andes D et al. . Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:503–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moretti-Branchini ML, Fukushima K, Schreiber AZ et al. . Trichosporon species infection in bone marrow transplanted patients. Diagn Microbiol Infect Dis 2001; 39:161–4. [DOI] [PubMed] [Google Scholar]
- 25.Matsue K, Uryu H, Koseki M et al. . Breakthrough trichosporonosis in patients with hematologic malignancies receiving micafungin. Clin Infect Dis 2006; 42:753–7. [DOI] [PubMed] [Google Scholar]
- 26.Bayramoglu G, Sonmez M, Tosun I et al. . Breakthrough Trichosporon asahii fungemia in neutropenic patient with acute leukemia while receiving caspofungin. Infection 2008; 36:68–70. [DOI] [PubMed] [Google Scholar]
- 27.Liao Y, Hartmann T, Zheng T et al. . Breakthrough trichosporonosis in patients receiving echinocandins: case report and literature review. Chin Med J 2012; 125:2632–5. [PubMed] [Google Scholar]
- 28.Anunnatsiri S, Chetchotisakd P, Mootsikapun P. Fungemia in non-HIV-infected patients: a five-year review. Int J Infect Dis 2009; 13:90–6. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto M, Takakura S, Hotta G et al. . Clinical characteristics and risk factors of non-Candida fungaemia. BMC Infect Dis 2013; 13:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hung CC, Chang SC, Chen YC et al. . Trichosporon beigelii fungemia in patients with acute leukemia: report of three cases. J Formos Med Assoc 1995; 94:127–31. [PubMed] [Google Scholar]
- 31.Liao Y, Yang S, Cong L et al. . In vitro activities of antifungal combinations against biofilms and planktonic forms of clinical Trichosporon asahii isolates. Antimicrob Agents Chemother 2014; 58:7615–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Bonaventura G, Pompilio A, Picciani C et al. . Biofilm formation by the emerging fungal pathogen Trichosporon asahii: development, architecture, and antifungal resistance. Antimicrob Agents Chemother 2006; 50:3269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinberger M, Leibovici L, Perez S et al. . Characteristics of candidaemia with Candida-albicans compared with non-albicans Candida species and predictors of mortality. J Hosp Infect 2005; 61:146–54. [DOI] [PubMed] [Google Scholar]
- 34.Garnacho-Montero J, Diaz-Martin A, Garcia-Cabrera E et al. . Impact on hospital mortality of catheter removal and adequate antifungal therapy in Candida spp. bloodstream infections. J Antimicrob Chemother 2013; 68:206–13. [DOI] [PubMed] [Google Scholar]
- 35.Velasco E, Bigni R. A prospective cohort study evaluating the prognostic impact of clinical characteristics and comorbid conditions of hospitalized adult and pediatric cancer patients with candidemia. Eur J Clin Microbiol Infect Dis 2008; 27:1071–8. [DOI] [PubMed] [Google Scholar]
- 36.Asmundsdottir LR, Erlendsdottir H, Gottfredsson M. Improving survival of patients with candidaemia: analysis of prognostic factors from a long-term, nationwide study in Iceland. Scand J Infect Dis 2005; 37:111–20. [DOI] [PubMed] [Google Scholar]
- 37.Liu CY, Huang LJ, Wang WS et al. . Candidemia in cancer patients: impact of early removal of non-tunneled central venous catheters on outcome. J Infect 2009; 58:154–60. [DOI] [PubMed] [Google Scholar]
- 38.Nucci M, Anaissie E, Betts RF et al. . Early removal of central venous catheter in patients with candidemia does not improve outcome: analysis of 842 patients from 2 randomized clinical trials. Clin Infect Dis 2010; 51:295–303. [DOI] [PubMed] [Google Scholar]
- 39.Nucci M, Anaissie E. Should vascular catheters be removed from all patients with candidemia? An evidence-based review. Clin Infect Dis 2002; 34:591–9. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez D, Park BJ, Almirante B et al. . Impact of early central venous catheter removal on outcome in patients with candidaemia. Clin Microbiol Infect 2007; 13:788–93. [DOI] [PubMed] [Google Scholar]
- 41.Liao Y, Zhao H, Lu X et al. . Efficacy of ethanol against Trichosporon asahii biofilm in vitro. Med Mycol 2015; 53:396–404. [DOI] [PubMed] [Google Scholar]
- 42.Hajjeh RA, Blumberg HM. Bloodstream infection due to Trichosporon beigelii in a burn patient: case report and review of therapy. Clin Infect Dis 1995; 20:913–6. [DOI] [PubMed] [Google Scholar]
- 43.Walsh TJ, Melcher GP, Rinaldi MG et al. . Trichosporon beigelii, an emerging pathogen resistant to amphotericin B. J Clin Microbiol 1990; 28:1616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toriumi Y, Sugita T, Nakajima M et al. . Antifungal pharmacodynamic characteristics of amphotericin B against Trichosporon asahii, using time-kill methodology. Microbiol Immunol 2002; 46:89–93. [DOI] [PubMed] [Google Scholar]
- 45.Marty FM, Barouch DH, Coakley EP et al. . Disseminated trichosporonosis caused by Trichosporon loubieri. J Clin Microbiol 2003; 41:5317–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anaissie EJ, Hachem R, Karyotakis NC et al. . Comparative efficacies of amphotericin B, triazoles, and combination of both as experimental therapy for murine trichosporonosis. Antimicrob Agents Chemother 1994; 38:2541–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walsh TJ, Lee JW, Melcher GP et al. . Experimental Trichosporon infection in persistently granulocytopenic rabbits: implications for pathogenesis, diagnosis, and treatment of an emerging opportunistic mycosis. J Infect Dis 1992; 166:121–33. [DOI] [PubMed] [Google Scholar]
- 48.Anaissie E, Gokaslan A, Hachem R et al. . Azole therapy for trichosporonosis: clinical evaluation of eight patients, experimental therapy for murine infection, and review. Clin Infect Dis 1992; 15:781–7. [DOI] [PubMed] [Google Scholar]
- 49.Nucci M, Marr KA. Emerging fungal diseases. Clin Infect Dis 2005; 41:521–6. [DOI] [PubMed] [Google Scholar]
- 50.Fournier S, Pavageau W, Feuillhade M et al. . Use of voriconazole to successfully treat disseminated Trichosporon asahii infection in a patient with acute myeloid leukaemia. Eur J Clin Microbiol Infect Dis 2002; 21:892–6. [DOI] [PubMed] [Google Scholar]
- 51.Serena C, Gilgado F, Marine M et al. . Efficacy of voriconazole in a guinea pig model of invasive trichosporonosis. Antimicrob Agents Chemother 2006; 50:2240–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hosokawa K, Yamazaki H, Mochizuki K et al. . Successful treatment of Trichosporon fungemia in a patient with refractory acute myeloid leukemia using voriconazole combined with liposomal amphotericin B. Transpl Infect Dis 2012; 14:184–7. [DOI] [PubMed] [Google Scholar]
- 53.Gabriel F, Noel T, Accoceberry I. Fatal invasive trichosporonosis due to Trichosporon loubieri in a patient with T-lymphoblastic lymphoma. Med Mycol 2011; 49:306–10. [DOI] [PubMed] [Google Scholar]
- 54.Paphitou NI, Ostrosky-Zeichner L, Paetznick VL et al. . In vitro antifungal susceptibilities of Trichosporon species. Antimicrob Agents Chemother 2002; 46:1144–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Falk R, Wolf DG, Shapiro M et al. . Multidrug-resistant Trichosporon asahii isolates are susceptible to voriconazole. J Clin Microbiol 2003; 41:911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li H, Lu Q, Wan Z et al. . In vitro combined activity of amphotericin B, caspofungin and voriconazole against clinical isolates of Trichosporon asahii. Int J Antimicrob Agents 2010; 35:550–2. [DOI] [PubMed] [Google Scholar]
- 57.Richardson MD. Changing patterns and trends in systemic fungal infections. J Antimicrob Chemother 2005; 56:i5–i11. [DOI] [PubMed] [Google Scholar]
- 58.Wolf DG, Falk R, Hacham M et al. . Multidrug-resistant Trichosporon asahii infection of nongranulocytopenic patients in three intensive care units. J Clin Microbiol 2001; 39:4420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grauer ME, Bokemeyer C, Bautsch W et al. . Successful treatment of a Trichosporon beigelii septicemia in a granulocytopenic patient with amphotericin B and granulocyte colony-stimulating factor. Infection 1994; 22:283–6. [DOI] [PubMed] [Google Scholar]
- 60.Walsh TJ, Newman KR, Moody M et al. . Trichosporonosis in patients with neoplastic disease. Medicine 1986; 65:268–79. [DOI] [PubMed] [Google Scholar]
- 61.Erer B, Galimberti M, Lucarelli G et al. . Trichosporon beigelii: a life-threatening pathogen in immunocompromised hosts. Bone Marrow Transplant 2000; 25:745–9. [DOI] [PubMed] [Google Scholar]
- 62.Gamaletsou MN, Walsh TJ, Zaoutis T et al. . A prospective, cohort, multicentre study of candidaemia in hospitalized adult patients with haematological malignancies. Clin Microbiol Infect 2014; 20:O50–7. [DOI] [PubMed] [Google Scholar]
- 63.Tashiro T, Nagai H, Kamberi P et al. . Disseminated Trichosporon beigelii infection in patients with malignant diseases: immunohistochemical study and review. Eur J Clin Microbiol Infect Dis 1994; 13:218–24. [DOI] [PubMed] [Google Scholar]
- 64.Lyman CA, Garrett KF, Pizzo PA et al. . Response of human polymorphonuclear leukocytes and monocytes to Trichosporon beigelii: host defense against an emerging opportunistic pathogen. J Infect Dis 1994; 170:1557–65. [DOI] [PubMed] [Google Scholar]
- 65.Ruhnke M, Rickerts V, Cornely OA et al. . Diagnosis and therapy of Candida infections: joint recommendations of the German Speaking Mycological Society and the Paul-Ehrlich-Society for Chemotherapy. Mycoses 2011; 54:279–310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.