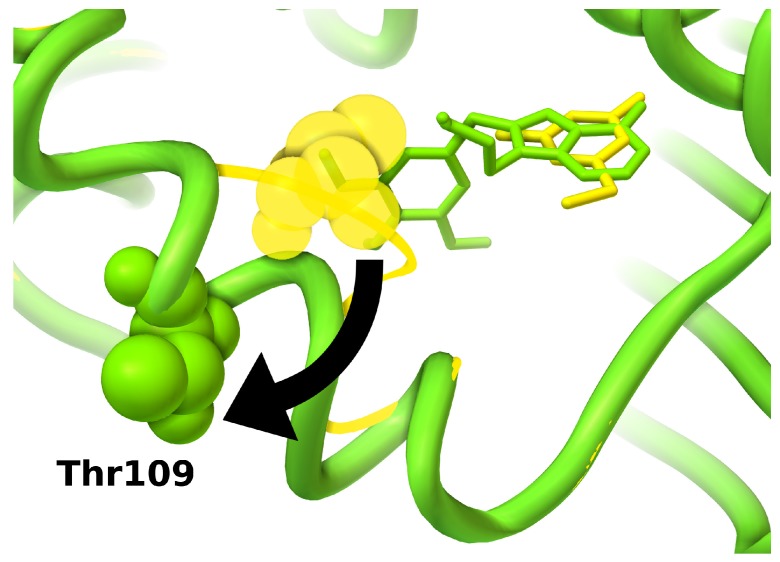
Figure 5.
Conformational re-arrangements in HSP90 induced by ATP site inhibitors. Protein secondary structures are shown as ribbons; conformations of key residue Thr109 are shown as spheres; ligands are shown as sticks. Small fragments can bind into a protein conformation similar to the apo conformation (yellow, PDB:2wi2) [134], in which an unwinded loop covers part of the binding site; larger ligands induce conformational rearrangements folding the loop in a helix conformation and uncovering a larger binding site (green, PDB:1uy6) [131].