Abstract
Disulfiram (DSF), used for the treatment of alcohol use disorders (AUDs) for over six decades, most recently has shown promise for treating cocaine dependence. Although DSF’s mechanism of action in alcohol abuse is due to the inhibition of liver mitochondrial aldehyde dehydrogenase (ALDH2), its mechanism of action in the treatment of cocaine dependence is unknown. DSF is a pro-drug, forming a number of metabolites each with discrete pharmacological actions. One metabolite formed during DSF bioactivation is S-(N, N-diethylcarbamoyl) glutathione (carbamathione) (carb). We previously showed that carb affects glutamate binding. In the present studies, we employed microdialysis techniques to investigate the effect of carb administration on dopamine (DA), GABA, and glutamate (Glu) in the nucleus accumbens (NAc) and medial prefrontal cortex (mPFC), two brain regions implicated in substance abuse dependence. The effect of DSF on DA, GABA, and Glu in the NAc also was determined. Both studies were carried out in male rats. Carb (20, 50, 200 mg/kg i v) in a dose-dependent manner increased DA, decreased GABA, and had a biphasic effect on Glu, first increasing and then decreasing Glu in both the NAc and mPFC. These changes all occurred concurrently. After carb administration, NAc and mPFC carb, as well as carb in plasma, were rapidly eliminated with a half-life for each approximately 4 min, while the changes in DA, GABA, and GLu in the NAc and mPFC persisted for approximately two hours. The maximal increase in carb (Cmax) in the NAc and mPFC after carb administration was dose-dependent, as was the area under the curve (AUC). DSF (200 mg/kg i p) also increased DA, decreased GABA, and had a biphasic effect on Glu in the NAc similar to that observed in the NAc after carb administration. When the cytochrome P450 inhibitor N-benzylimidazole (NBI) (20 mg/kg i p) was administered before DSF dosing, no carb could be detected in the NAc and plasma and also no changes in NAc DA, GABA, and GLu occurred. Changes in these neurotransmitters occurred only if carb was formed from DSF. When NBI was administered prior to dosing with carb, the increase in DA, decrease in GABA, and biphasic effect on GLu was similar to that seen after dosing with carb only. The i p or i v administration of carb showed similar changes in DA, GABA, and GLu, except the time to reach Cmax for DA as well as the changes in GABA, and GLu after i p administration occurred later. The elimination half-life of carb and the area under the curve (AUC) were similar after both routes of administration. It is concluded that carb must be formed from DSF before any changes in DA, GABA, and GLu in the NAc and mPFC are observed. DSF and carb, when administered to rats, co-release DA, GABA, and GLu. Carb, once formed can cross the blood brain barrier and enter the brain. Although inhibition of liver ALDH2 is the accepted mechanism for DSF’s action in treating AUDs, the concurrent changes in DA, GABA, and GLu in the NAc and mPFC after DSF administration suggest that changes in these neurotransmitters as a potential mechanism of action not only for AUDs, but also for cocaine dependence cannot be excluded.
Keywords: Disulfiram, Carbamathione, Microdialysis, Dopamine, GABA, Glutamate
1. Introduction
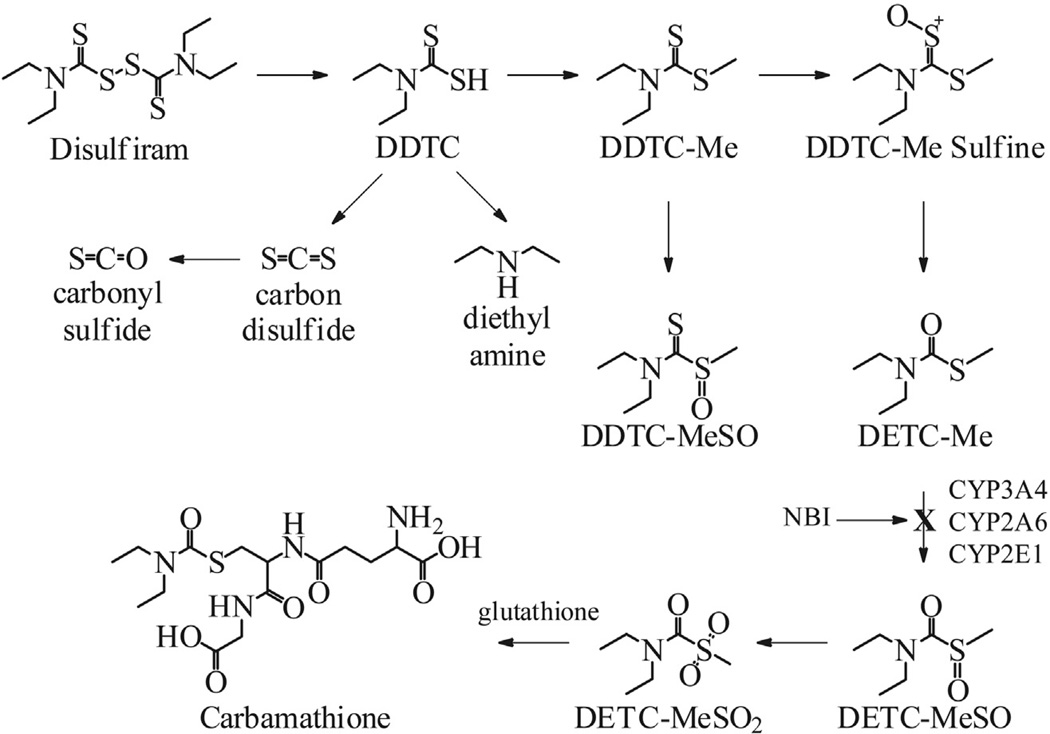
Disulfiram (DSF), accidentally discovered approximately 65 years ago, has been used since then as a deterrent for alcohol use disorders (AUDs) (Hald and Jacobson, 1948). Clinical studies also have shown DSF to be a useful drug candidate for the treatment of cocaine use (Carroll et al., 1998, 2000; George et al., 2000; Petrakis et al., 2000; Carroll et al., 2004). Recently, DSF also has shown promise in the treatment of non-substance abuse disorders such as pathological gambling (Reuter et al., 2005). At this time other than for AUDs, there are no pharmacotherapies currently approved by any regulatory agency for the treatment of cocaine use or pathological gambling disorders. Although DSF’s mechanism of action in treating AUDs is established as being due to its inhibition of liver mitochondrial aldehyde dehydrogenase (ALDH2), the mechanism of action of DSF in treating cocaine dependence or pathological gambling is unknown, but suggests a central mechanism. Seminal studies (Yourick and Faiman, 1989, 1991) showed DSF to be a pro-drug that requires bioactivation, and a number of metabolites are formed each with discrete pharmacological properties. The chemical pathways, the metabolites formed, including the metabolite S-methyl N, N-diethylthiolcarbamate sulfoxide (DETC-MeSO) which is responsible for ALDH2 inhibition which is responsible for the anti-alcohol effect (Hart and Faiman, 1992), and the respective cytochrome P450 and other enzymes identified in DSF’s bioactivation have been described previously in a comprehensive series of studies (Fig. 1) (Madan et al., 1993; Hart and Faiman, 1993; Madan and Faiman, 1994a; Madan and Faiman, 1994b, 1995; Madan et al., 1994; Madan and Faiman, 1995; Madan et al., 1995, 1998).
Fig. 1.
Disulfiram bioactivation. DDTC: diethyldithiocarbamate. DDTC-Me: diethyldithiocarbamate methyl ester. DDTC-MeSO: diethyldithiocarbamate methyl ester sulfoxide. DETC-Me: S-methyl N, N-diethylthiolcarbamate. DETC-MeSO: S-methyl N, N-diethylthiolcarbamate sulfoxide. DETC-MeSO2: S-methyl N, N-diethylthiolcarbamate sulfone. NBI: N-benzylimidazole.
The finding that DSF was effective independent of concurrent alcohol use (Carroll et al., 2004), and coupled with previous studies that carb does not inhibit ALDH2 (Jin et al., 1994; Nagendra et al., 1997), suggested DSF’s mechanism of action for the treatment of cocaine dependence must be due to a metabolite further downstream from DETC-MeSO (Fig. 1). Since the concentration of reduced glutathione (GSH) in vivo is high (1–6 mM) (Potter and Tran, 1993), the DSF metabolites DETC-MeSO and S-methyl N, N-diethylthiolcarbamate sulfone (DETC-MeSO2) (Jin et al., 1994; Nagendra et al., 1997) rapidly carbamoylate the sulfhydryl of glutathione to S-(N, N-diethylcarbamoyl) glutathione, the metabolite of interest and is referred to as carbamathione (carb) (Schloss, 2007) (Fig. 1).
In earlier studies, we showed that the i v administration of carb to mice irreversibly blocks glutamate binding to mouse brain synaptic membranes (Nagendra et al., 1997), and speculated that carb may affect both the N-methyl-d-aspartate (NMDA) and non-NMDA glutamate receptor subtypes. NMDA receptor antagonists are known to increase brain DA (Cano-Cebrian et al., 2003). Non-competitive NMDA receptor antagonists (Thomson et al., 1985) also increase the release of glutamate (GLu) from the PFC (Moghaddam et al., 1997; Del Arco and Mora, 2002; Lorrain et al., 2003) and NAc (Razoux et al., 2007), and DA from the PFC (Moghaddam et al., 1997; Lorrain et al., 2003). The glutamatergic system also modulates DA in the NAc (Imperato et al., 1990a, 1990b; Youngren et al., 1993; Taber et al., 1996; Sesack et al., 2003). Pharmacological modulation of the GABAergic system in cocaine-treated rats affects extracellular DA in the NAc. In addition, several studies reported that GABA-transaminase inhibition increase GABA and DA (Gerasimov et al., 2001; Dewey et al., 1998; Morgan and Dewey, 1998; Pan et al., 2012). Both DA and GLu also have been implicated in cocaine dependence (Kalivas, 2007). Thus, DA, GABA, and GLu appear inter-related in the addictive process for cocaine, and possibly other drugs of abuse, as those studies as well as others (Tzschentke and Schmidt, 2003; Spanagel and Kiefer, 2008; Uys and LaLumiere, 2008) have shown.
Cocaine dependence, as well as other substance abuse disorders is considered to be a brain disease (Leshner and Koob, 1999). Thus, efforts have been made to identify psychoactive agents that affect the dopaminergic, GABAergic, and glutamatergic pathways, and evaluate their potential in treating cocaine dependence (Preti, 2007; Gass and Olive, 2008; Karila et al., 2008; Shorter and Kosten, 2011; Somaini et al., 2011). Unfortunately, the clinical effectiveness of these agents is inconsistent (Vocci and Ling, 2005; Pierce and Kumaresan, 2006; Preti, 2007; Kampman, 2010; Shorter and Kosten, 2011). Given that DSF’s efficacy in treating cocaine dependence appears independent of ALDH2 inhibition (Carroll et al., 2004), that carb seems to have an effect on GLu binding (Nagendra et al., 1997), and the inter-dependent relationship between DA, GABA, and GLu (Sesack et al., 2003; Lapish et al., 2006; Del Arco and Mora, 2008), we hypothesized that the DSF metabolite carb may be responsible for the increase in brain DA after DSF administration, and also possible changes in GABA and GLu. To test this hypothesis we carried out microdialysis studies to determine carb’s effect on these neurotransmitters in the NAc and mPFC, two brain regions central to the addictive process (McFarland and Kalivas, 2001). DA, GABA and GLu were determined concurrently since all three neurotransmitters have been implicated in cocaine dependence (Kalivas, 2007).
2. Methods
2.1. Chemicals, reagents, and drugs
All chemicals, reagents, and solutions used for the analysis of DA, GABA, and GLu, have been described previously (Kaul et al., 2010, 2011). Carb was synthesized using the method of Jin et al. (1994) with modification (Schloss, 2007). DSF and N-benzylimidazole (NBI) were purchased from Sigma–Aldrich, St. Louis, MO, USA).
2.2. Analytical procedures
Analysis of NAc and mPFC DA, GABA, GLu and carb in microdialysis samples in the carb dose–response studies (Figs. 2–4) were carried out utilizing a previously developed method employing micellar electrokinetic chromatography with laser-induced fluorescence (MEKC-LIF) (Kaul et al., 2011) which measured DA, GABA, and GLu simultaneously in both the NAc and mPFC (Kaul et al., 2011). The detection and quantification of carb in the microdialysis samples from the plasma was determined concomitantly with these neurotransmitters using recently developed LC MS/MS techniques (Kaul et al., 2010).
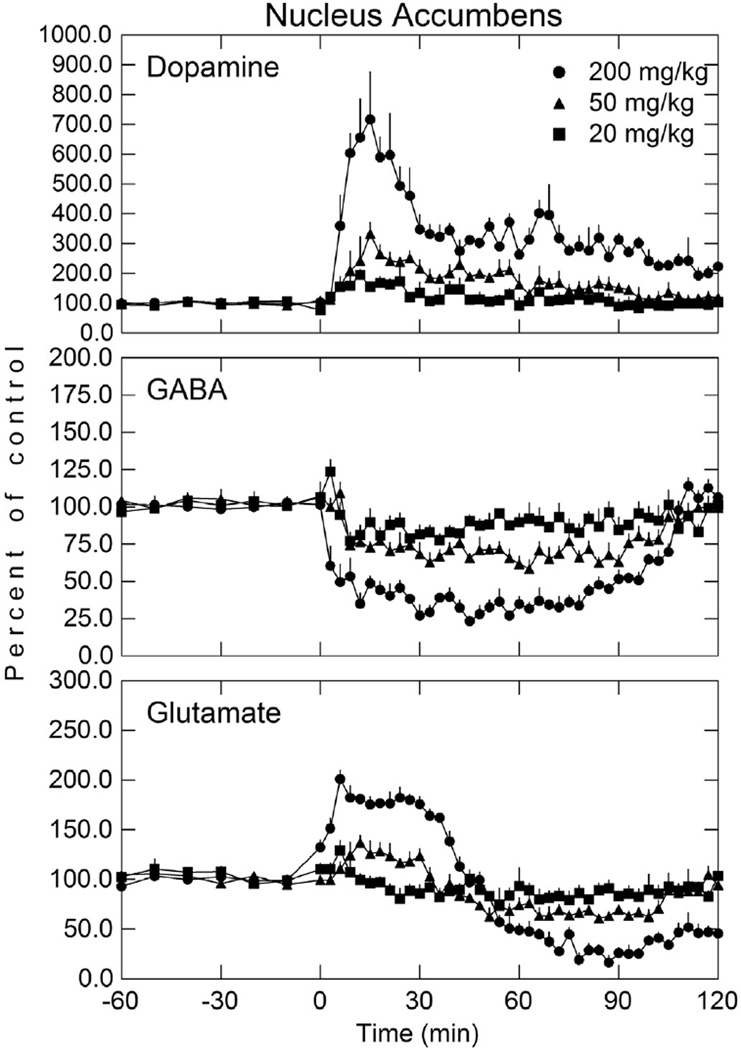
Fig. 2.
Dopamine, GABA, and glutamate concentration in the NAc after various doses of i v carbamathione. Perfusion of NAc was initiated 60 min before the i v administration of carbamathione (t = 0). Microdialysis samples were collected every 3 min for neurotransmitter analysis. The basal concentrations of dopamine, GABA, and glutamate in microdialysis samples from the NAc were 7.4 ± 1.8 nmol/L, 63.5 ± 11.7 nmol/L, and 2.9 ± 0.5 µmol/L, respectively. Data shown as a percent of baseline (± SEM; n = 5). See Table 1 for statistical analysis.
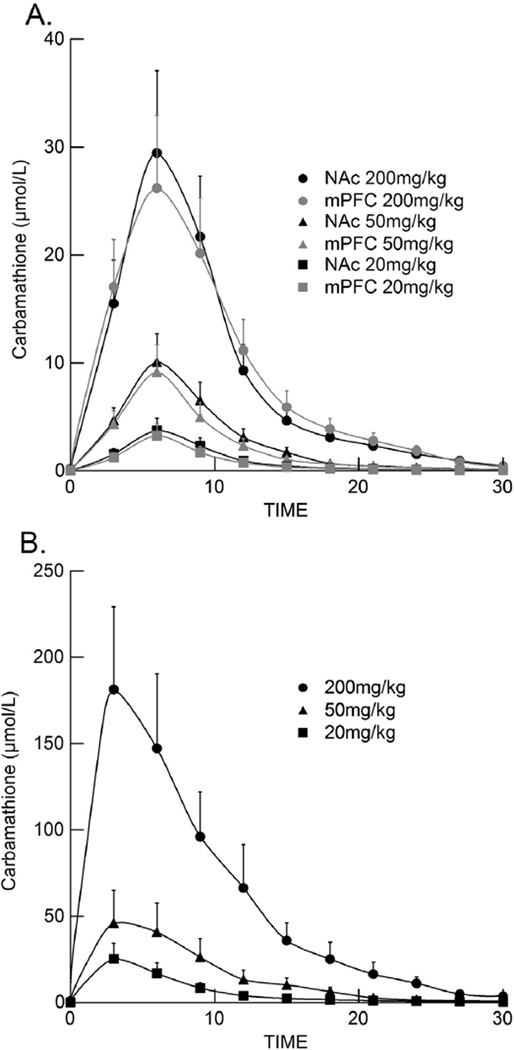
Fig. 4.
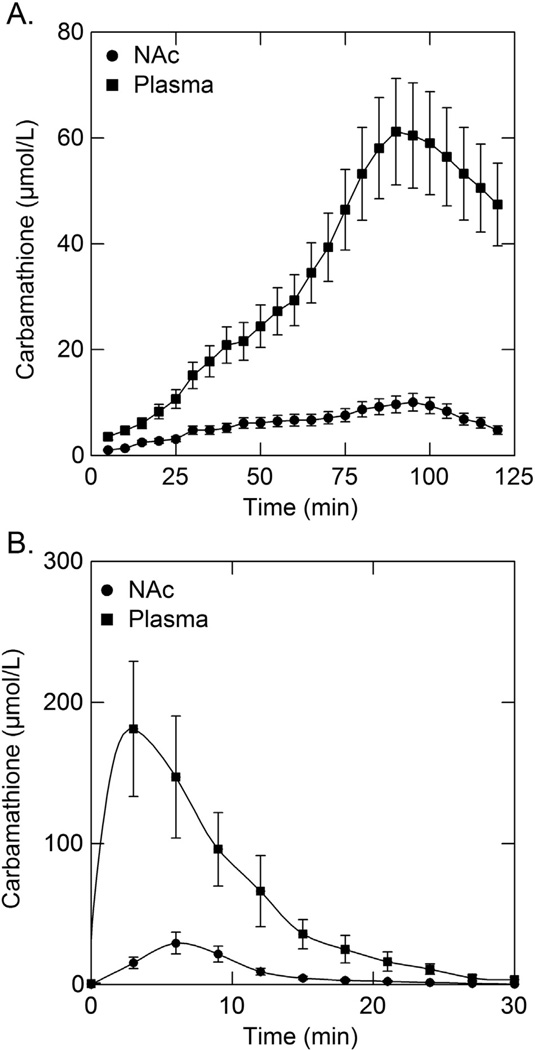
Carbamathione concentration in the NAc, mPFC and plasma as a function of time after i v carbamathione administration. Data depict the mean carbamathione concentration (± SEM; n = 5) in the (A) NAc, mPFC and (B) plasma following carbamathione administration (20, 50, 200 mg/kg i v). Microdialysis samples were collected every 3 min.
2.3. Animals, surgeries, and microdialysis studies
Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 300 g to 400 g were used. Rats were housed in a temperature and humidity controlled animal facility maintained on a 12 h light/dark cycle with access to food and water ad libitum. Animals were brought into the laboratory from the animal facility, kept individually in their cage, and acclimated for 24 h before studies were initiated. Experiments were conducted during the light phase. Prior to surgery, the rats were initially anesthetized by isofluorane inhalation, followed by a subcutaneous injection of a mixture of ketamine (67.5 mg/kg), xylazine (3.4 mg/kg) and acepromazine (0.67 mg/kg).
Rats were implanted with an i v catheter for the carb or an intraperitoneal (i p) catheter for the DSF drug-dosing studies followed by the vascular and brain microdialysis probes. For i v drug-dosing, a PE-10 cannula (Fisher Scientific, Pittsburgh, PA) was implanted into the external femoral vein, with the cannula exiting the skin of the rat at the back of the neck between the shoulders (Kaul et al., 2011). For the DSF i p studies, a recently developed route of administration was employed which minimized animal handling since neurotransmitter concentrations can change as a result of stress associated with animal handling. This technique avoided this possibility. A 5–10 cm of PE-50 cannula (Fisher Scientific, Pittsburgh, PA) was inserted into the abdominal cavity so that the end of the cannula lay between the small intestine and the ventral cavity wall. The cannula was held in place by suturing it to the edge of the opening in the muscle wall. The muscle incision was sutured and the free end of the cannula was externalized through an incision at the back of the neck between the shoulders. This i p technique allowed the drugs to be administered to the rats without the need to handle each animal multiple times for drug-dosing.
The dialysis brain probes for determining the neurotransmitters in the shell of the NAc and in the mPFC were implanted as follows. Holes 1 mm in diameter were drilled through the skull at the insertion sites and intra-cerebral guide cannulas were lowered into the specified regions using a micromanipulator attached to the stereotaxic apparatus. The guide cannulas were positioned 2 mm above the specified regions and then affixed to the skull with dental cement. The dummy probe in the guide cannula was replaced with microdialysis probes (CMA 12 Elite) with 2 mm membranes purchased from CMA Microdialysis (North Chelmsford, MA). The coordinates of the insertion sites relative to the bregma line were +1.5 mm anterior, +0.9 mm lateral and −6.2 mm ventral for the NAc shell site and +3.7 mm anterior, +0.7 mm lateral and −1.0 mm ventral for the mPFC (Paxinos and Watson, 1986). After the surgical procedures, the rats were administered 0.5–3 ml/kg of saline subcutaneously to prevent dehydration. A heating pad was placed under the rats to provide warmth during recovery from anesthesia. Plasma carb samples were obtained from a microdialysis probe implanted into the jugular vein utilizing vascular probes that were fabricated in-house. After surgical implantation, the brain and vascular probes were perfused with aCSF and Ringer’s solution respectively at a rate of 2 µL/min. The perfusion and collection of three minute samples in the microdialysis dose–response studies after carb administration or five minute collection times after DSF was initiated 24 h after surgery. This same post-surgery time period before initiation of the studies has been used successfully by others (Devoto et al., 2012).
2.4. Microdialysis probe calibration
Microdialysis samples were collected using a CMA 100 micro infusion pump and a HoneyComb fraction collector (Bioanalytical Systems Inc., West Lafayette, IN). The characteristics of the implanted microdialysis probes were evaluated at the end of each experiment. The relative recovery of carb through the microdialysis probes was estimated by delivery experiments (Song and Lunte, 1999) by perfusing 1 µmol/L carb through the microdialysis probes in vivo at 2 µL/min and determining the percentage that perfused through the membrane. The in vivo extraction efficiency (mean ± SEM) for carb was determined to be 25.8 ± 4.1% for the brain probes and 57.5 ± 10.4% for the plasma vascular probes. The concentration of carb determined in the microdialysis samples was corrected for the extraction efficiency of the probe used. The in vitro extraction efficiency (mean ± SEM) for DA, GABA, and GLu was estimated to be 14.1 ± 1.9%,17.2 ± 1.8%, and 13.5 ± 1.4% respectively. The concentrations of DA, GABA, and GLu were expressed as percent (mean ± SEM) of baseline concentrations in order to monitor changes from basal levels after either carb or DSF administration.
2.5. Drug dosing
Carb (20, 50, and 200 mg/kg i v) was prepared in sodium bicarbonate solution (Kaul et al., 2010). DSF was prepared as a suspension in saline solution and then sonicated, and administered at a dose of 200 mg/kg i p. NBI was prepared in saline with a few drops of 0.1 mol/L HCl added and the solution sonicated. The dose of NBI administered was 20 mg/kg i p (Hart and Faiman, 1993). In the studies with carb, microdialysis sampling was carried out every three minutes. In these dose–response studies, a sample size of five rats per dose of carb was used. After all probes were in place in each rat, perfusion with aCSF was initiated 60 min before the i v administration of carb. At this time neurotransmitter concentrations were stable (Figs. 2 and 3). Carb was then administered i v for each dose of carb and microdialysate samples collected every three minutes for two hours. This procedure was repeated for a total of five rats for each dose of carb studied which constituted the sample size. For the DSF experiments (Figs. 6 and 7), this dosing procedure was repeated except that in the DSF or DSF plus NBI studies the drugs were given i p using the newly developed technique, and microdialysate sampling carried out every five minutes. This procedure was repeated for a total of three rats for each dose of DSF or DSF plus NBI which constituted the sample size.
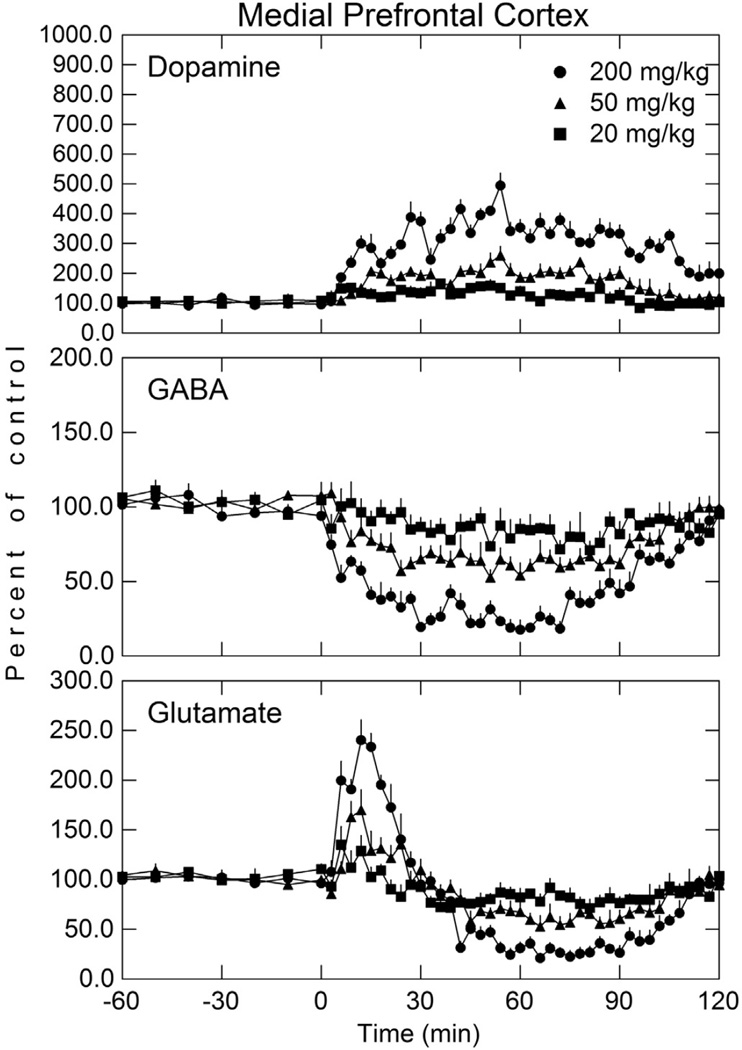
Fig. 3.
Dopamine, GABA, and glutamate concentration in the mPFC after various doses of i v carbamathione. Perfusion of mPFC was initiated 60 min before the i v administration of carbamathione (t = 0). Microdialysis samples were collected every 3 min for neurotransmitter analysis. The basal concentrations of dopamine, GABA, and glutamate in microdialysis samples from the mPFC were 3.8 ± 0.5 nmol/L, 49.8 ± 11.7 nmol/L, and 2.4 ± 0.3 µmol/L, respectively. Data shown as a percent of baseline (± SEM; n = 5). See Table 1 for statistical analysis.
Fig. 6.
Dopamine, GABA, and glutamate levels in the NAc following disulfiram (200 mg/kg; i p) administration. Perfusion of NAc was initiated 60 min before the i p administration of disulfiram (t = 0). Microdialysis samples were collected at 5 min intervals for neurotransmitter analysis. Data shown as mean percent of baseline (± SEM; n = 3).
Fig. 7.
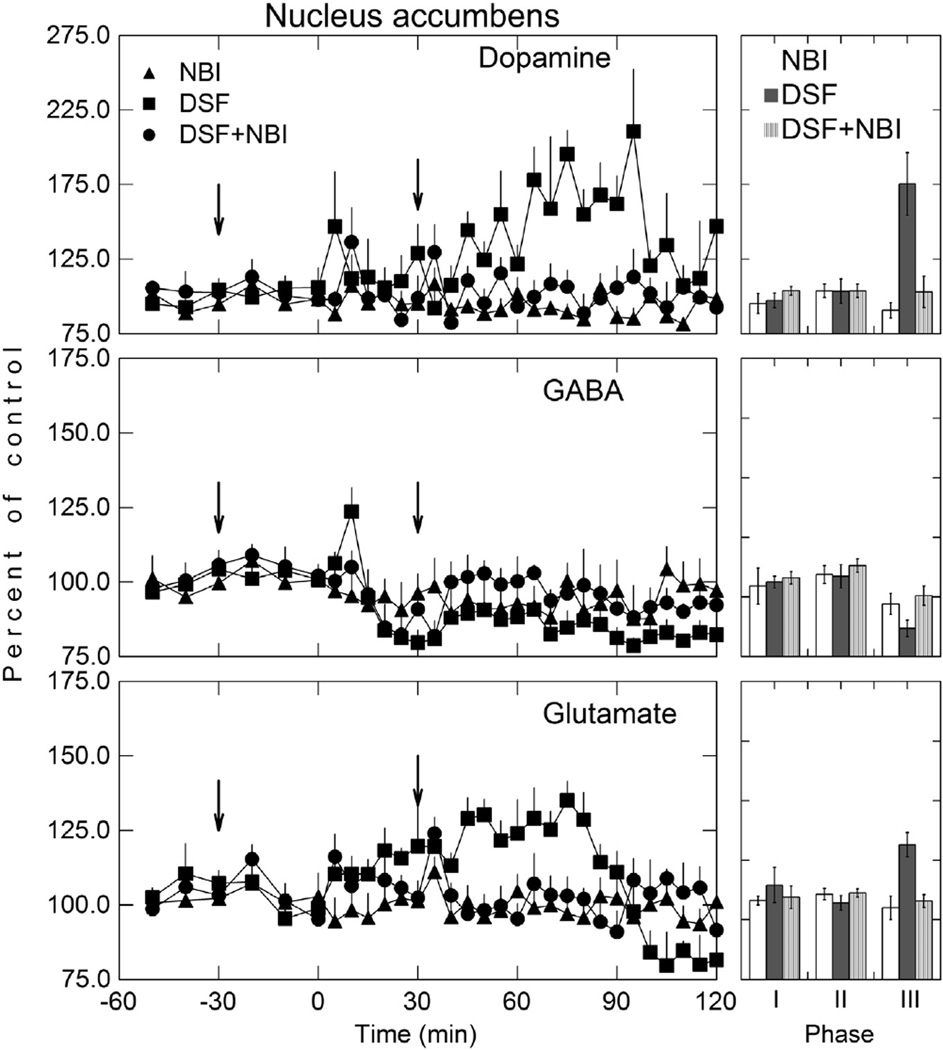
Dopamine, GABA, and glutamate in the NAc after inhibition of disulfiram metabolism. NBI (20 mg/kg; i p) was administered 30 min before disulfiram (200 mg/kg; i p) and a booster dose given 30 min after disulfiram (see arrows). Perfusion of NAc was initiated 60 min before the i v administration of disulfiram (t = 0). Arrows are the time at which NBI was administered. Microdialysis samples were collected at 5 min intervals for neurotransmitter analysis. Data shown as mean percent of baseline (± SEM; n = 3). Phase I represents a measure of the baseline 30 min before NBI treatment, Phase II reflects the first 30 min after NBI administration, and Phase III is the region of maximal dopamine, GABA, and glutamate response. See Table 2 for statistical comparisons.
2.6. Histology
At the completion of each experiment, the rats were killed by placing the animal in an isofluorane chamber for 30 min. Rat brains were harvested for histological confirmation of brain probe position. The rat brains were fixed in buffered formalin (10%). The tissues were embedded in paraffin wax and the sections cut at 5 µm and stained with hematoxylin-eosin. Only probes with at least 85% of the active dialysis membrane in the NAc shell or mPFC were included in the study.
3. Animal use
All animal experiments were conducted during the light phase and in accordance with guidelines established by the Institutional Animal Care and Use Committee at the University of Kansas and in accordance with NIH approved guidelines and met AAALAC standards.
4. Data and statistical analysis
In Figs. 2–8, data are given as mean ± SEM. Values for the first five consecutive samples were averaged to calculate basal neurotransmitter levels. In the carb and DSF studies, the start of drug administration (time point zero) was corrected for the lag time of the microdialysis system. For the carb dose–response studies, statistical and post-hoc analysis were performed with two-way repeated measures analysis of variance (RM ANOVA) to compare neurotransmitter levels between treatment doses (between-group factor) and treatment over time (repeated measures factor). For the DSF plus NBI inhibition studies, a one-way ANOVA was performed separately for each neurotransmitter (DA, GABA, GLu) (Table 3) and phase (I, II, III) (Fig. 7) to determine if drug treatments were significantly different from each other. Each phase refers to the time period when measurements were obtained. Phase I was 30 min before any drug treatment, Phase II was 30 min after the first NBI treatment, and Phase III was the 35 min period within the region of the maximal DA response. When phase-related one-way ANOVAs were significant, post-hoc tests were carried out (Bonferroni) to determine if the DSF treatment was significantly different from the other two treatments. The α level for statistical significance was set at p < 0.05 for all comparisons. When assumptions for homoscedasticity were not supported, ANOVAs were based on log10 transformations of the data.
Fig. 8.
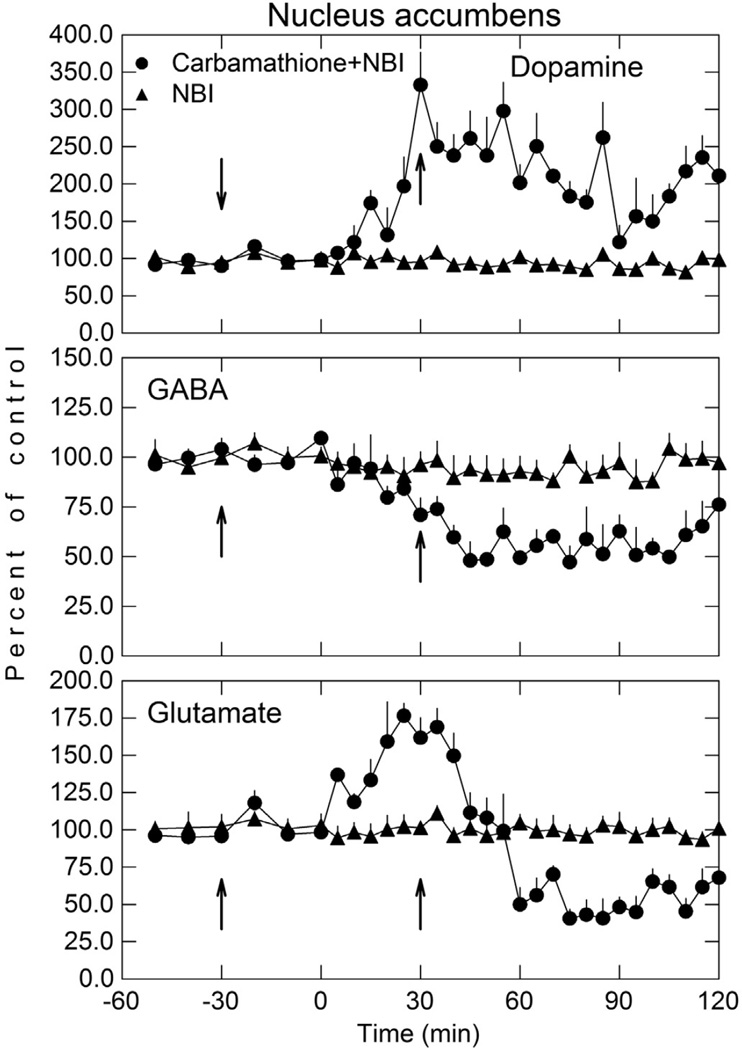
Dopamine, GABA, and glutamate in the NAc after carbamathione administration with and without NBI. NBI (20 mg/kg; i p) was administered 30 min before carbamathione (200 mg/kg; i p) and a booster dose of NBI given 30 min after carbamathione (see arrows). Perfusion of NAc was initiated 60 min before the i v administration of carbamathione (t = 0). Microdialysis samples were collected at 5 min intervals for neurotransmitter analysis. Data shown as mean percent of baseline (± SEM; n = 3). A RM ANOVA indicated carbamathione + NBI was significantly different from the NBI only group (p < 0.001) for DA, GABA, and Glu).
Table 3.
Summary of analysis of variance results for disulfiram-NBI studies. One-way ANOVA was performed for each phase (I–III).
| Measure | Analysis of variance effect | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Phase I | Phase II | Phase III | |||||||
| df | F | p | df | F | p | df | F | p | |
| NAc DA | 2,6 | 0.76 | ns | 2,6 | 0.09 | ns | 2,6 | 12.05 | <0.05 |
| NAc GABA | 2,6 | 0.16 | ns | 2,6 | 0.38 | ns | 2,6 | 3.29 | ns |
| NAc GLu | 2,6 | 0.40 | ns | 2,6 | 0.82 | ns | 2,6 | 9.80 | <0.05 |
5. Results
5.1. Carbamathione and NAc dopamine, GABA, and glutamate
Carb (20 mg/kg, 50 mg/kg, and 200 mg/kg i v) increased DA 96%, 232%, and 616%, (p < 0.05) respectively in the NAc shell (Fig. 2), with the maximal increase occurring approximately 15 min after drug administration. Extracellular GABA decreased 23%, 42%, and 77%, respectively (p < 0.05) at these three doses, with the maximal decrease occurring approximately 45 min after carb administration. GLu in the NAc exhibited a biphasic effect. Initially, GLu increased 29%, 37% and 101% (p < 0.05) after each dose of carb, remained elevated for approximately 39 min, after which GLu then decreased 26%, 39%, and 84%, respectively (p < 0.05) below basal levels.
5.2. Carbamathione and mPFC dopamine, GABA, and glutamate
In the mPFC (Fig. 3), after i v carb (20 mg/kg 50 mg/kg and 200 mg/kg) administration, extracellular DA increased 66%, 159%, and 395% respectively (p < 0.05), although the increase in DA was less than that seen in the NAc. Carb also decreased extracellular GABA 29%, 47%, and 82% at these three doses (p < 0.05). As observed in the NAc, carb also produced a biphasic effect on extracellular GLu in the mPFC. Initially, extracellular GLu in the mPFC increased 35%, 70%, and 140% (p < 0.05) respectively at the three doses, and remained elevated for approximately 30 min, after which GLu then decreased of 29%, 47%, and 79% below basal levels for approximately one hour after drug administration before returning to base-line values. These changes in GABA and GLu were similar to those found in the NAc. As observed in the NAc, the greater the dose of carb administered, the greater the change in DA, GABA, and GLu in the mPFC.
Of interest was that the peak increase in DA in the NAc at all three doses occurred approximately 15 min after carb dosing whereas in the mPFC (Fig. 3) the peak effect occurred approximately 50 min after drug administration. Furthermore the peak DA concentration reached in the NAc was greater than that in the mPFC. The time for the maximal decrease in GABA and times for the changes in GLu were similar to those in the NAc.
5.3. Post-hoc analysis of carbamathione on NAc and mPFC dopamine, GABA, and glutamate
Post-hoc analysis (Table 1) of the effect of these doses (treatment-dose) on DA in the NAc showed that the effect of 200 mg/kg of carb was significantly different than from a dose of 50 mg/kg and 20 mg/kg but the 50 mg/kg dose was not statistically different from the 20 mg/kg dose. This was the same for GLu. For GABA, post-hoc analysis found statistically significant differences between all three doses of carb. In the mPFC, post-hoc analysis showed a statistically significant increase in DA and a decrease in GABA at all three doses. For GLu, only the 50 mg/kg versus the 20 mg/kg dose showed no statistically significant difference (Table 1). The lack of statistical significance between the 50 mg/kg and 20 mg/kg dose after carb administration for GLu in the NAc and mPFC appears to be due to the greater variability in neurotransmitter concentration and limited sample size.
Table 1.
Summary of analysis of variance results for carbamathione dose–response studies. The table lists the ANOVA results for the main effect of dose. Statistical and post-hoc analysis were performed using a two-way repeated measures ANOVA.
| Measurea | Analysis of variance effectb | ||
|---|---|---|---|
| Dose | |||
| df | F | p | |
| NAc DA | 2,6 | 37.15 | <0.01 |
| NAc DA (200 mg vs 50 mg CARB) | 1,4 | 91.79 | <0.01 |
| NAc DA (50 mg vs 20 mg CARB) | 1,4 | 6.87 | ns |
| NAc DA (200 mg vs 20 mg CARB) | 1,4 | 58.56 | <0.01 |
| NAc GABA | 2,6 | 148.36 | <0.01 |
| NAc GABA (200 mg vs 50 mg CARB) | 1,4 | 104.64 | <0.01 |
| NAc GABA (50 mg vs 20 mg CARB) | 1,4 | 77.46 | <0.01 |
| NAc GABA (200 mg vs 20 mg CARB) | 1,4 | 209.58 | <0.01 |
| NAc GLu | 2,6 | 27.54 | <0.01 |
| NAc GLu (200 mg vs 50 mg CARB) | 1,4 | 23.54 | <0.01 |
| NAc GLu (50 mg vs 20 mg CARB) | 1,4 | 5.15 | ns |
| NAc GLu (200 mg vs 20 mg CARB) | 1,4 | 37.44 | <0.01 |
| mPFC DA | 2,6 | 265.08 | <0.01 |
| mPFC DA (200 mg vs 50 mg CARB) | 1,4 | 501.92 | <0.01 |
| mPFC DA (50 mg vs 20 mg CARB) | 1,4 | 53.03 | <0.01 |
| NAc DA (2000 mg vs 20 mg CARB) | 1,4 | 394.46 | <0.01 |
| mPFC GABA | 2,6 | 147.09 | <0.01 |
| mPFC GABA (200 mg vs 50 mg CARB) | 1,4 | 133.17 | <0.01 |
| mPFC GABA (50 mg vs 20 mg CARB) | 1,4 | 22.72 | <0.01 |
| NAc GABA (200 mg vs 20 mg CARB) | 1,4 | 231.13 | <0.01 |
| mPFC GLu | 2,6 | 37.69 | <0.01 |
| mPFC GLu (200 mg vs 50 mg CARB) | 1,4 | 92.05 | <0.01 |
| mPFC GLu (50 mg vs 20 mg CARB) | 1,4 | 1.69 | ns |
| NAc GLu (200 mg vs 20 mg CARB) | 1.4 | 57.23 | <0.01 |
Post-hoc tests are in italics.
Significant probabilities are in bold font.
5.4. Concentration–time profile of carbamathione in NAc, mPFC, and plasma
A comparison of the concentration–time profile between carb in the NAc, mPFC, and in the plasma after each i v dose of drug is given in Fig. 4A and B respectively. The peak concentration of carb in the NAc and mPFC occurred after 5 min, and in the plasma approximately 3 min after carb administration at the respective doses and then decreased rapidly. The rapid loss of carb in the NAc and mPFC (Fig. 4A) and in plasma (Fig. 4B) was in contrast to the changes in extracellular DA, GABA and GLu observed in the NAc (Fig. 2) and mPFC (Fig. 3). In those brain regions the increase in DA, decrease in GABA, and the biphasic effect that occurred with GLu were maintained for at least two hours. This suggests that there is no relationship between the concentration of carb in the NAc, mPFC or in plasma and the effect of carb on DA, GABA, and GLu in these brain regions.
5.5. Pharmacokinetic parameters
The various pharmacokinetic parameters for carb in the NAc, mPFC, and in plasma are given in Table 2. There was a direct relationship between the i v dose of carb administered and the carb Cmax and AUC in both the NAc and mPFC, and in the plasma. The carb tmax, t1/2, and Kelim were similar in both the NAc and mPFC and in plasma irrespective of the dose of carb administered, suggesting a diffusion process and a first-order mechanism.
Table 2.
Pharmacokinetic parameters after carbamathione dosing. The pharmacokinetic parameters after carbamathione administration (20, 50, and 200 mg/kg i v; n = 5) in the mPFC, NAc, and plasma are listed below. Microdialysis samples were collected every 3 min for carbamathione analysis. Data are expressed as mean ± SEM.
| Dose | Cmax | tmax | t1/2 | Kelim | AUC |
|---|---|---|---|---|---|
| 200 mg/kg mPFC | 26.2 ± 6.5 | 4.5 ± 1.5 | 4.15 ± 0.37 | 0.17 ± 0.02 | 273 ± 66 |
| 200 mg/kg NAc | 29.4 ± 7.3 | 4.5 ± 1.5 | 4.10 ± 0.48 | 0.16 ± 0.02 | 270 ± 67 |
| 200 mg/kg Plasma | 182 ± 47 | 1.5 ± 1.5 | 4.67 ± 0.41 | 0.15 ± 0.03 | 1854 ± 458 |
| 50 mg/kg mPFC | 9.24 ± 2.64 | 4.5 ± 1.5 | 4.14 ± 0.58 | 0.17 ± 0.03 | 71.1 ± 21.3 |
| 50 mg/kg NAc | 10.1 ± 3.0 | 4.5 ± 1.5 | 4.05 ± 0.57 | 0.17 ± 0.02 | 84.1 ± 25.2 |
| 50 mg/kg Plasma | 45.9 ± 9.4 | 1.5 ± 1.5 | 4.37 ± 0.44 | 0.16 ± 0.03 | 447 ± 124 |
| 20 mg/kg mPFC | 3.25 ± 0.43 | 4.5 ± 1.5 | 4.17 ± 0.67 | 0.17 ± 0.03 | 23.7 ± 6.3 |
| 20 mg/kg NAc | 3.72 ± 0.54 | 4.5 ± 1.5 | 4.09 ± 0.71 | 0.17 ± 0.03 | 29.1 ± 8.1 |
| 20 mg/kg Plasma | 25.2 ± 8.3 | 1.5 ± 1.5 | 4.34 ± 0.55 | 0.16 ± 0.04 | 183 ± 70 |
mPFC = Medial prefrontal cortex.
NAc = Nucleus accumbens.
Cmax = maximum carbamathione concentration (µmol/L).
tmax = time to maximum concentration (min).
t1/2 = elimination half-life (min).
Kelim = elimination constant (1/min).
AUC = area under the dialysate concentration versus time curve (µmol/L min).
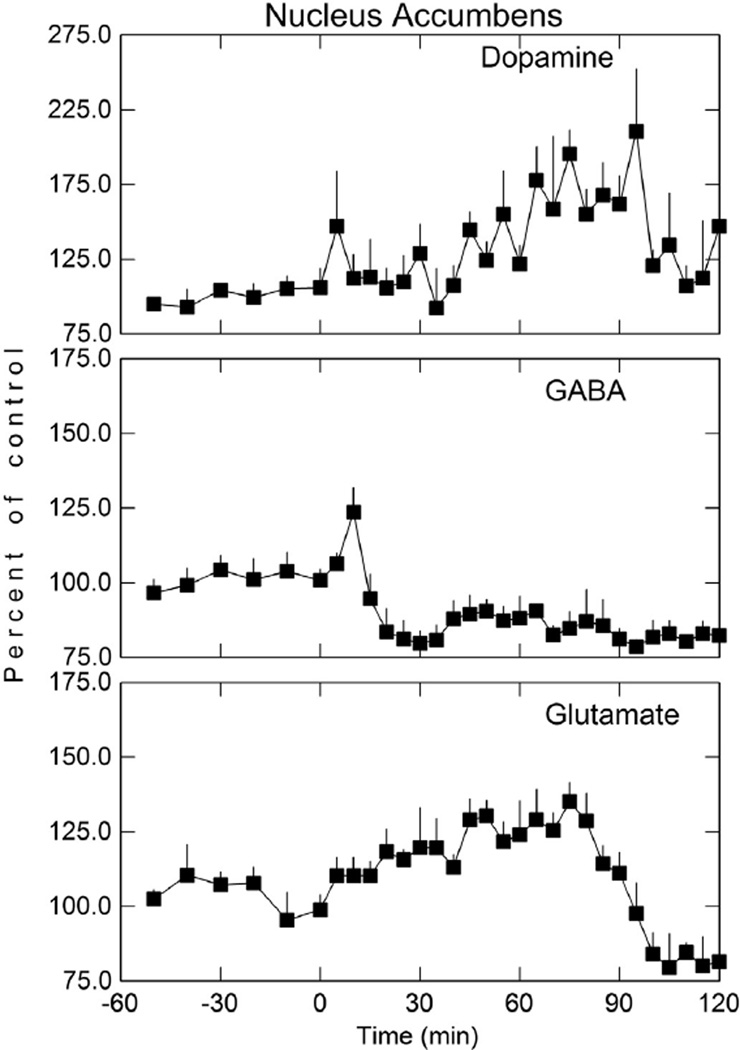
5.6. Disulfiram and NAc dopamine, GABA, and glutamate
After DSF (200 mg/kg i p) administration, carb was found in the NAc (Fig. 5A). The concentration of carb in the NAc was less than the concentration of carb found in the NAc after i v carb (Fig. 5B). Similarly, carb in plasma after DSF (Fig. 5A) was less than the concentration of carb in plasma after carb administration (Fig. 5B). The time for carb to reach the maximal concentration (tmax) after DSF was greater (90 min) than after carb (5 min) dosing. These differences would be expected since carb was given i v whereas DSF was administered i p which requires DSF to be absorbed and then metabolized to form carb (Fig. 1).
Fig. 5.
Effect of disulfiram and carbamathione administration on NAc and plasma carbamathione. Data depict the mean carbamathione concentration (± SEM) in the NAc (circles) and plasma (squares) following (A) disulfiram (n = 3) or (B) carbamathione (n = 5) administration. Microdialysis samples were collected every 3 min for carbamathione and 5 min for disulfiram.
DSF also produced an increase in NAc DA, decrease in GABA, and a biphasic effect on GLu first increasing and then decreasing GLu in the extracellular fluid (Fig. 6) similar to that observed in the NAc after carb administration (Fig. 2). DSF increased DA in the NAc, remained elevated for approximately 100 min and reached a maximal increase of approximately 111% (p< 0.05) over baseline. Although there was an initial increase in GABA in the extracellular fluid of the NAc within the first 10 min, GABA then decreased and remained at a reduced level for approximately two hours after DSF administration. GLu in the NAc exhibited a biphasic effect. GLu increased initially with a peak increase of 35% (p < 0.05) over baseline that occurred approximately 75 min after DSF dosing after which GLu decreased below basal levels. The maximal GLu decrease was 20% (p < 0.05) which occurred approximately 120 min after DSF administration. The peak concentration of DA, the maximal decrease in GABA, and the maximal increase and decrease in GLu (Fig. 6) after DSF was less than that observed after carb administration (Fig. 2) as would be expected. The changes in DA, GABA, and GLu in the NAc after an acute dose of DSF (Fig. 6) was similar to that found after carb administration (Fig. 2) except that the concentration of these neurotransmitters after DSF dosing was lower. This is most likely due to less carb formed from DSF and the dose-dependent effect that carb has on these neurotransmitters (Fig. 2).
5.7. Inhibition of disulfiram metabolism with N-benzylimidazole and NAc dopamine, GABA, and glutamate
The hypothesis that the change in DA, GABA, and GLu in the NAc (Fig. 2) after DSF administration is due to formation of carb was tested using the general cytochrome P450 inhibitor NBI to inhibit DSF metabolism. NBI (20 mg/kg i p) was given to rats 30 min before DSF administration and a booster dose of NBI given 30 min after DSF administration in order to maintain inhibition of DSF metabolism (Hart and Faiman, 1993). The effect of the inhibition of DSF metabolism on DA, GABA, and GLu in the shell of the NAc is given in Fig. 7.
The concentration of DA, GABA, and GLu in the NAc between the NBI only (control group) and the NBI plus DSF group were not statistically different (p < 0.05) (Phase III). That is, NBI pretreatment blocked DSF-induced changes in these neurotransmitters. Although there was a minimal increase early in DA, GABA, and GLu in the NBI plus DSF treatment group, this increase was not statistically significant. In rats treated with DSF, carb was detected in both the NAc and plasma (Fig. 5). However, in rats treated with NBI and then given DSF, no carb could be detected in either the NAc or plasma. These findings suggest that in order for a DSF-induced change in DA, GABA, and GLu to occur, carb must first be formed. A summary of one-way ANOVA comparisons in NAc DA, GABA, and GLu during all three phases of the experimental protocol for the DSF plus NBI studies (Fig. 7) is given in Table 3. During phase I, which is a measure of neurotransmitter base-line values 30 min before NBI treatment, no statistical differences were observed. Similarly, there were no statistical differences during phase II, which reflects the first 30 min after NBI treatment. Phase III, which is the region of maximal DA, GABA, and GLu response, statistically significant differences were apparent only with DA and GLu. Although the trend for GABA was in right direction, the small sample size may have contributed to the lack of any statistical significance in the GABA group.
5.8. Inhibition of carbamathione metabolism by N-benzylimidazole and NAc dopamine, GABA, and glutamate
NBI administration prior to carb dosing was investigated to determine if the inhibition of liver drug metabolizing enzymes had any effect on the carb-induced changes in DA, GABA, and GLu in the NAc once carb was formed (Fig. 8). In these studies carb was administered i p rather than given i v as in the dose–response studies (Fig. 2). Thus the effect of inhibition of the drug metabolism as well as comparing i p versus i v administration could be assessed. NBI had no effect on carb’s induced increase DA, decrease in GABA, and biphasic effect on GLu in the NAc, as the change observed in these neurotransmitters was similar to that observed after carb administration without NBI dosing (Fig. 2) An analysis of variance comparing DA, GABA, and GLu showed there was no statistical significance between the carb group (positive control) and NBI plus carb group. These findings suggest that once carb is formed, inhibition of liver cytochrome P450 enzymes has no effect on the basal levels of DA, GABA, and GLu. After i p carb administration, the values for the increase in DA, decrease in GABA and biphasic effect on GLu in the NAc are about half of that seen after i v carb administration (Fig. 2). This would be expected since absorption and distribution of carb to the brain must first occur after i p administration compared to a direct effect after i v administration. Since carb given i p produced changes in DA, GABA, and GLu in the NAc, these findings also suggest that first pass effects don’t play any role in carb’s ability to affect the vesicular release of these neurotransmitters.
6. Discussion
The finding that carb increased DA, decreased GABA, and had a biphasic effect on GLu in both the NAc and mPFC concurrently, is the first time this has been reported for a DSF metabolite. The mechanism by which carb produced these simultaneous changes in DA, GABA, and GLu in both the NAc and mPFC (Figs. 2 and 3) is unclear at this time. Since carb affects glutamate binding (Nagendra et al., 1997), the present studies were carried out to test the hypothesis that carb could affect DA, GABA, and GLu because of the interaction between these neurotransmitters, each of which can modulate their respective release (Sesack et al., 2003; Pierce and Kumaresan, 2006). Furthermore, the co-release of DA and GLu also has been suggested previously (Trudeau, 2004; Lapish et al., 2006). The increase in DA in the NAc and mPFC we observed after carb (Figs. 2 and 3) and DSF (Fig. 6) is similar to the findings from microdialysis studies by Vaccari et al. (1996) in which DSF and diethyldithiocarbamate (DDTC) (Fig. 1) increased the release of vesicular DA. Those investigators proposed that this was due to an interaction with the DA carrier and increased membrane permeability. However, from studies in progress (Levant and Faiman, unpublished results) we have observed that carb has no effect on the DA transporter. Vaccari et al. (1998), employing microdialysis techniques also found that DSF and DDTC increased the release of vesicular GLu from the neostriatum. Since DSF is metabolized to carb (Fig. 1), it is more than likely that any changes in DA and GLu observed by those investigators most likely can be attributed to carb. Vaccari et al. (1998) did not observe a biphasic effect on GLu in contrast to our findings with carb given either i v (Figs. 2 and 3) or i p (Fig. 8), or DSF (Fig. 6).
The concentration of DA in the extracellular fluid of the NAc after carb administration (Fig. 2) was greater than that found in the mPFC (Fig. 3). Furthermore, the time to reach the maximal increase in DA in the NAc after carb administration was approximately 15 min compared to approximately 50 min in the mPFC These findings are consistent with the findings from in vivo fast-scan cyclic voltammetry studies by Garris and Wightman (1994). Those studies showed that the initial rate of release of DA into the extracellular space was some 8-fold higher in the NAc than in the mPFC suggesting regional specific DA release characteristics. It is possible that the greater tonic DA concentration observed in the NAc compared to the mPFC arises from differences in phasic release. Although voltammetry and microdialysis data may not reflect the same phenomenon, our results parallel the findings of Garris and Wightman (1994). This also could explain the difference for the biphasic effect of GLu observed between results for carb (Figs. 2 and 3) and DSF (Fig. 6) and the DSF and DDTC findings of Vaccari et al. (1998).
The concentration of carb in the NAc and mPFC increased in a dose-dependent manner (Fig. 4), and peaked within 5 min in both these brain regions regardless of the dose of carb administered, and was undetectable after 30 min. In plasma, carb peaked after 2.5 min and was undetectable after 12 min after a low dose and 26 min after a high dose of carbamathione (Fig. 4). However, the changes in DA, GABA and GLu in both the NAc and mPFC persisted for almost one to two hours (Figs. 2 and 3) long after carb could be detected in these brain regions (Fig. 4). This suggests that carb may either affect the vesicular storage and release of these neurotransmitters over a prolonged period of time and recovery is slow while carb is rapidly removed, or that a metabolite of carb is responsible for the observed effect on these neurotransmitters. Studies in progress suggest the latter may be the case. The half-life for carb disappearance from the NAc, mPFC, or plasma are all similar, this being approximately 4 min regardless of the dose of carb administered (Table 2) suggesting first-order kinetics and consistent with passive transfer. There was a direct relationship between the area under the curve (AUC) for carb and the dose of carb administered (Table 2) which again is consistent with first order kinetics.
DSF, the parent compound of carb, increased carb in the NAc and plasma (Fig. 5) and also increased NAc DA, decreased GABA, and had a biphasic effect on GLu (Fig. 6) similar to that observed after carb administration (Fig. 2). That the increase in DA after DSF is due to carb formation is supported by several lines of evidence. After DSF administration, carb appeared in both the NAc and plasma (Fig. 5A), and in addition there was an increase in NAc DA, decrease in GABA and a biphasic effect on GLu (Fig. 6) similar to that observed after carb administration (Fig. 2). The Cmax for carb in plasma and in the NAc after DSF administration (Fig. 5A) occurred after approximately 90 min whereas after carb administration (Fig. 5B), the Cmax in the NAc occurred within 5 min and in plasma within 12 min. The differences between DSF and carb administration (Fig. 5A and B) is because DSF was given i p whereas carb, the active metabolite was given i v. The i p administration of DSF requires absorption, distribution and metabolism of the drug which explains the reason for the lag time before any changes in DA, GABA, and GLu occur (Fig. 6). The DSF inhibition studies with NBI provide further evidence that the increase in brain DA is due to carb and not the DSF metabolite DDTC as previously proposed (Goldstein et al., 1964). When DSF metabolism was inhibited by pretreatment with NBI, there was no statistical difference in DA, GABA, and GLu in the NAc between the NBI only-treated group (positive control) and the NBI plus DSF group (Fig. 7). Furthermore, no carb could be detected either in the NAc or in plasma in rats treated with NBI and then administered DSF. Thus, if no carb is formed, no changes in DA, GABA, and GLu were observed (Fig. 7). A comparison between i p DSF (Fig. 6) and i p carb (Fig. 8) further supports carb as the active metabolite responsible for the changes in DA, GABA, and GLu. The percentage of carb formed from DSF is unknown but is presumed to be small since Gessner and Jakubowski (1972) estimated that 0.05% of DDTC is converted to DDTC-Me, a precursor to carb (Fig.1). Carb’s effect on NAc and mPFC DA, GABA, and GLu is dose-dependent (Figs. 2 and 3). Thus, the change in concentration of these neurotransmitters formed after DSF administration (Fig. 6) is smaller when compared to carb (Fig. 8). Reduction of DSF to DDTC is dependent upon glutathione reductase ((Strömme, 1963a; Cobby et al., 1977) and not the cytochrome P450 enzymes. The CYP 3A4, 2E1 and 2A6 enzymes responsible for DSF bioactivation and carb formation (Madan et al., 1995) are necessary after formation of DDTC (Fig. 1). If DDTC is the metabolite responsible for the increase in DA after DSF administration as proposed (Goldstein et al., 1964), there should have been an increase in DA in the NAc after DSF administration in the NBI-treated rats since inhibition of the CYP enzymes by NBI occurs downstream from DDTC (Fig. 1). Therefore, once DDTC is formed, further metabolism to carb cannot proceed. In vivo, DDTC is rapidly methylated in both rodents (Gessner and Jakubowski, 1972), Cobby et al., 1977) and in humans ((Faiman et al., 1984), and demethylation of DDTC-Me to DDTC is unlikely (Gessner and Jakubowski, 1972). Additional evidence that the formation of carb from DSF is required for any changes in these in these neurotransmitters is seen from the observation that the NAc/plasma ratio after i v carb (Fig. 4) is approximately 0.16 which is similar to that for the NAc/plasma ratio after DSF administration (Fig. 5A,), even though the route of administration was different, i v versus i p. Finally, that the CYP P450 enzymes are not involved in the metabolism of carb after it is formed from DSF is apparent from the findings that carb increased DA, decreased GABA, and had a biphasic effect on GLu in the NAc even when rats are first treated with NBI and then administered carb (Fig. 8). In addition, the pharmacokinetic parameters t1/2, Kelm, and AUC for carb with and without NBI pretreatment were similar providing further support that after carb is formed the CYP P450 enzymes no longer play a role in carb’s effect on changes in DA, GABA, and GLu. An important finding is that carb, a glutathione conjugate, given i p crosses the blood brain barrier and affects DA, GABA, and GLu in the NAc (Fig. 8). Carb (200 mg/kg) was administered both i v and i p. Yet, the AUC, and t1/2 for both routes of administration were similar. Only the Cmax and tmax were different with the i p route taking longer to produce its effect which is expected.
From a clinical perspective, estimates suggest that adults reduce approximately 50 g of DSF within a 24 h period (Strömme, 1963b). This is a large dose of DSF compared to the standard 250 mg daily dose used clinically in treating alcohol or cocaine dependence. Thus, after the usual 250 mg clinical dose of DSF, little DSF should remain and any DDTC formed would be expected to be rapidly metabolized to several metabolites (Gessner and Jakubowski, 1972; Johansson, 1992; Madan et al., 1993, 1994) with little DDTC remaining. This is supported by human studies as less than 10 ng/ml of DDTC is found in plasma after a single dose of DSF (Scappaticci et al., 1990) and 0.7 µg/ml of DDTC found in plasma after 12 consecutive days of dosing with DSF (Faiman et al., 1984).
These studies show for the first time that the DSF metabolite carb simultaneously increased DA, decreased GABA and increased and then decreased GLu in both the NAc and mPFC in a dose-dependent manner. Although it has been suggested that corelease of these neurotransmitters may occur in various disease states that implicate monoamine pathways (Trudeau, 2004), these findings are the first report that DSF as a result of carb formation can co-release these neurotransmitters. When the metabolism of DSF was inhibited, no carb was detected in brain and plasma, and no change in DA, GABA, and GLu occurred in the NAc.
An increase in brain DA and change in the DA/NE ratio due to DβH inhibition by DSF has been proposed as contributing to cocaine dependence ((Gaval-Cruz et al., 2008; Gaval-Cruz and Weinshenker, 2009; Schroeder et al., 2010). Although this concept may still be valid, the increase in DA appears to be due to carb and not DSF. We did not determine the effect of carb on DβH activity. However, carb is not a good drug candidate for inhibiting DβH (Hogarth, 2012) as is DDTC, which is the metabolite of DSF responsible for the inhibition of this enzyme (Goldstein et al., 1964). Furthermore, in very preliminary in vitro studies we found that carb does not bind to copper, the proposed mechanism by which DSF, through its metabolite DDTC complexes with the copper of DβH and inhibits this enzyme (Goldstein et al. (1964). As for DSF’s efficacy in cocaine dependence or non-substance abuse disorders such as pathological gambling, the co-release of DA, GABA, and GLu and their role cannot be discounted considering carb’s simultaneous effect on these neurotransmitters. The neurocircuitry in the reward pathway in the brain associated with these neurotransmitters is such that each can influence the release of the other (McFarland and Kalivas, 2001; Del Arco and Mora, 2008). Focusing only on the role of either DA, GABA, or GLu individually in cocaine dependence, or other substance abuse disorders (Leshner and Koob, 1999), may only provide limited information. Of interest is that in studies by Yao et al. (2010), those investigators found that ALDH2 inhibition attenuates cocaine self-administration and prevents cocaine or cue-induced reinstatement in a rat model of cocaine relapse. The molecular mechanism proposed is that ALDH2 inhibition increases tetrahydropapaveroline (THP) formation through the condensation of 3,4-dihydroxyphenylacetaldehyde (DOPAL) and DA forming THP, which through a negative-feedback mechanism reduces DA production. Although this is an interesting concept, carb does not inhibit ALDH2 (Jin et al., 1994; Nagendra et al., 1997), yet carb increases DA in the NAc and PFC (Figs. 2 and 3). Studies into carb’s mechanism of action in increasing DA are needed. Also, the present studies were carried out in naïve rats and the effect of carb in cocaine or any other substance abuse-dependent animal model both biochemically and behaviorally is unknown and needs to be determined.
7. Conclusions
DSF is a pro-drug and can be metabolized to a number of metabolites. The finding that the metabolite carb increased DA, decreased GABA, and had a biphasic effect on GLu in both the NAc and mPFC, and that these neurotransmitters are co-released represents a novel finding for this DSF metabolite. Inhibition of DSF metabolism prevented the formation of carb and attenuated the changes in DA, GABA, and GLu. Thus, carb must be formed in order for DSF to affect these neurotransmitters. The identification of carb as a modulator of DA, GABA, and GLu in the NAC and mPFC provides an opportunity for carb to be used as a pharmacological tool to better understand the mechanism of action of DSF, not only in cocaine dependence but other substance abuse disorders. In light of DSF’s effect on DA, GABA, and GLu in the NAc and mPFC, a central action for DSF in treating alcohol and cocaine dependence as well as non-substance-related dependencies cannot be excluded.
Acknowledgments
This work was supported in part by a grant from the National Institute on Drug Abuse (NIDA), National Institutes of Health and Human Services (DA 021727 to MDF.). The authors also wish to acknowledge Dr. Michael Thompson of the pathology laboratory at Lawrence Memorial Hospital for his assistance with the rat brain histology, to Dr. Gillian Whitaker for her assistance with analyzing the plasma samples, and to Dr. Stephen C. Fowler for assistance with the statistical analysis.
Footnotes
The authors declare no conflicts of interest.
References
- Cano-Cebrian MJ, Zornoza-Sabina T, Guerri C, Polache A, Granero L. Local acamprosate modulates dopamine release in the rat nucleus accumbens through NMDA receptors: an in vivo microdialysis study. Naunyn Schmiedebergs Arch. Pharmacol. 2003;367:119–125. doi: 10.1007/s00210-002-0674-3. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, Rounsaville BJ. Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Arch. Gen. Psychiatry. 2004;61:264–272. doi: 10.1001/archpsyc.61.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Ball SA, McCance E, Frankforter TL, Rounsaville BJ. One-year follow-up of disulfiram and psychotherapy for cocaine-alcohol users: sustained effects of treatment. Addiction. 2000;795:335–1349. doi: 10.1046/j.1360-0443.2000.95913355.x. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Ball SA, McCance E, Rounsavile BJ. Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction. 1998;93:713–727. doi: 10.1046/j.1360-0443.1998.9357137.x. [DOI] [PubMed] [Google Scholar]
- Cobby J, Mayersohn M, Selliah S. The rapid reduction of disulfiram in blood and plasma. J. Pharmacol. Exp. Ther. 1977;202:724–731. [PubMed] [Google Scholar]
- Del Arco A, Mora F. NMDA and ampa/kainate glutamatergic agonists increase the extracellular concentrations of GABA in the prefrontal cortex of the freely moving rat: modulation by endogenous dopamine. Brain Res. Bull. 2002;57:623–630. doi: 10.1016/s0361-9230(01)00758-4. [DOI] [PubMed] [Google Scholar]
- Del Arco AD, Mora F. Prefrontal cortex-nucleus accumbens interaction: in vivo modulation by dopamine and glutamate in the prefrontal cortex. Pharmacol. Biochem. Behav. 2008;90:226–235. doi: 10.1016/j.pbb.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Devoto P, Flore G, Saba P, Cadeddu R, Gessa GL. Disulfiram stimulates dopamine release from noradrenergic terminals and potentiates cocaine-induced dopamine release in the prefrontal cortex. Psychopharmacol. (Berl.) 2012;219:1153–1164. doi: 10.1007/s00213-011-2447-5. [DOI] [PubMed] [Google Scholar]
- Dewey SL, Morgan AE, Ashby CR, Horan B, Kushner SA, Logan J, Volkow MD, Fowler JS, Gardner EL, Brodie JD. A novel strategy for the treatment of cocaine addiction. Synapse. 1998;30:119–129. doi: 10.1002/(SICI)1098-2396(199810)30:2<119::AID-SYN1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Faiman MD, Jensen JC, LaCoursiere RB. Elimination kinetics of disulfiram in alcoholics after single and repeated doses. Clin. Pharmacol. Ther. 1984;36:520–526. doi: 10.1038/clpt.1984.213. [DOI] [PubMed] [Google Scholar]
- Garris PA, Wightman RM. Different kinetics govern dopaminergic transmission in the amygdala, prefrontal cortex, and striatum: an in vivo voltammetric study. J. Neurosci. 1994;14:442–450. doi: 10.1523/JNEUROSCI.14-01-00442.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem. Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaval-Cruz M, Schroeder JP, Liles LC, Javors MA, Weinshenker DM. Effects of disulfiram and dopamine beta-hydroxylase knockout on cocaine-induced seizures. Pharmacol. Biochem. Behav. 2008;89:556–562. doi: 10.1016/j.pbb.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaval-Cruz M, Weinshenker D. Mechanisms of disulfiram-induced cocaine abstinence: antabuse and cocaine relapse. Mol. Interv. 2009;9:175–187. doi: 10.1124/mi.9.4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George T, Chawarski MC, Pakes J, Carroll KM, Kosten TR, Schottenfeld RS. Disulfiram versus placebo for cocaine dependence in buprenorphine-maintained subjects: a preliminary trial. Biol. Psychiatry. 2000;47:1080–1086. doi: 10.1016/s0006-3223(99)00310-8. [DOI] [PubMed] [Google Scholar]
- Gerasimov MR, Schiffer WK, Gardner EL, Marsteller DA, Lennon IC, Taylor SJ, Brodie JD, Ashby CR, Dewey Sl. GABAergic blockade of cocaine associated cue-Induced increases in nucleus accumbens dopamine. Eur. J. Pharmacol. 2001;414:205–209. doi: 10.1016/s0014-2999(01)00800-7. [DOI] [PubMed] [Google Scholar]
- Gessner T, Jakubowski M. Diethyldithiocarbamic acid methyl ester: a metabolite of disulfiram. Biochem. Pharmacol. 1972;21:219–230. doi: 10.1016/0006-2952(72)90272-9. [DOI] [PubMed] [Google Scholar]
- Goldstein M, Anagnoste B, Lauber E, McKereghan MR. Inhibition of dopamine-beta-hydroxylase by disulfiram. Life Sci. 1964;3:763–767. doi: 10.1016/0024-3205(64)90031-1. [DOI] [PubMed] [Google Scholar]
- Hald J, Jacobson E. A drug sensitizing the organism to ethyl alcohol. Lancet. 1948;2:1001–1004. doi: 10.1016/s0140-6736(48)91514-1. [DOI] [PubMed] [Google Scholar]
- Hart BW, Faiman MD. In vitro and in vivo inhibition of rat liver aldehyde dehydrogenase by s-methyl n, n-diethylthiolcarbamate sulfoxide, a new metabolite of disulfiram. Biochem. Pharmacol. 1992;43:403–406l. doi: 10.1016/0006-2952(92)90555-w. [DOI] [PubMed] [Google Scholar]
- Hart BW, Faiman MD. Bioactivation of s-methyl n, n diethylthiolcarbamate to s-methyl n, n-diethylthiolcarbamate sulfoxide: implications for the role of cytochrome P450. Biochem. Pharmacol. 1993;12:2285–2290. doi: 10.1016/0006-2952(93)90619-8. [DOI] [PubMed] [Google Scholar]
- Hogarth G. Metal-dithiocarbamate complexes: chemistry and biological activity. Mini Rev. Med. Chem. 2012;12:1202–1215. doi: 10.2174/138955712802762095. [DOI] [PubMed] [Google Scholar]
- Imperato A, Honore T, Jensen LH. Dopamine release in the nucleus caudatus and in the nucleus accumbens is under glutamatergic control through non-NMDA receptors: a study in freely-moving rats. Brain Res. 1990a;530:223–228. doi: 10.1016/0006-8993(90)91286-p. [DOI] [PubMed] [Google Scholar]
- Imperato A, Scrocco MG, Bacchi S, Angelucci L. NMDA receptors and in vivo dopamine release in the nucleus accumbens and caudatus. Eur. J. Pharmacol. 1990b;187:555–556. doi: 10.1016/0014-2999(90)90387-l. [DOI] [PubMed] [Google Scholar]
- Jin L, Davis MR, Hu P, Baillie TA. Identification of novel glutathione conjugates of disulfiram and diethyldithiocarbamate in rat bile by liquid chromatography–tandem mass spectrometry. Evidence for metabolic activation of disulfiram in vivo. Chem. Res. Toxicol. 1994;7:526–533. doi: 10.1021/tx00040a008. [DOI] [PubMed] [Google Scholar]
- Johansson B. A review of the pharmacokinetics and pharmacodynamics of disulfiram and its metabolites. Acta Psychiatr. Scand. 1992;86:15–26. doi: 10.1111/j.1600-0447.1992.tb03310.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Neurobiology of cocaine addiction: implications for new pharmacotherapy. Am. J. Addict. 2007;16:71–78. doi: 10.1080/10550490601184142. [DOI] [PubMed] [Google Scholar]
- Kampman KM. What’s new in the treatment of cocaine addiction. Curr. Psychiatry Rep. 2010;12:441–447. doi: 10.1007/s11920-010-0143-5. [DOI] [PubMed] [Google Scholar]
- Karila L, Gorelick D, Weinstein A, Noble F, Benyamina A, Coscas S, Blecha L, Lowenstein W, Martinot JL, Reynaud M, Lepine JP. New treatments for cocaine dependence: a focused review. Int. J. Neuropsychopharmacol. 2008;11:425–438. doi: 10.1017/S1461145707008097. [DOI] [PubMed] [Google Scholar]
- Kaul S, Faiman MD, Lunte CE. Determination of GABA, glutamate and carbamathione in brain microdialysis samples by capillary electrophoresis with fluorescence detection. Electrophoresis. 2011;32:284–291. doi: 10.1002/elps.201000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul S, Williams TD, Lunte CE, Faiman MD. LC-MS/MS determination of carbamathione in microdialysis samples from rat brain and plasma. J. Pharm. Biomed. Anal. 2010;51:186–191. doi: 10.1016/j.jpba.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapish CC, Seamans JK, Chandler LJ. Glutamate-dopamine cotransmission and reward processing in addiction. Alcohol Clin. Exp. Res. 2006;30:1451–1465. doi: 10.1111/j.1530-0277.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- Leshner AI, Koob GF. Drugs of abuse and the brain. Proc. Assoc. Am. Physicians. 1999;111:99–108. doi: 10.1046/j.1525-1381.1999.09218.x. [DOI] [PubMed] [Google Scholar]
- Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA. Effects of ketamine and n-methyl-d-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience. 2003;117:697–706. doi: 10.1016/s0306-4522(02)00652-8. [DOI] [PubMed] [Google Scholar]
- Madan A, Parkinson A, Faiman MD. Role of flavin-dependent monooxygenases and cytochrome p450 enzymes in the sulfoxidation of s-methyl n, n-diethylthiolcarbamate. Biochem. Pharmacol. 1993;12:2291–2297. doi: 10.1016/0006-2952(93)90620-c. [DOI] [PubMed] [Google Scholar]
- Madan A, Faiman MD. NADPH-dependent regioselective s-oxidation of a thionosulfur-and thioether-containing xenobiotic, diethyldithiocarbamate methyl ester by rat liver microsomes. Drug Metab. Dispos. 1994a;22:324–330. [PubMed] [Google Scholar]
- Madan A, Faiman MD. Diethyldithiocarbamate methyl ester sulfoxide, an inhibitor of rat liver mitochondrial low Km aldehyde dehydrogenase and putative metabolite of disulfiram. Alcohol Clin. Exp. Res. 1994b;18:1013–1017. doi: 10.1111/j.1530-0277.1994.tb00075.x. [DOI] [PubMed] [Google Scholar]
- Madan A, Williams TD, Faiman MD. Glutathione and glutathione-s-transferase-dependent oxidative desulfuration of the thione xenobiotic diethyldithiocarbamate methyl ester. Mol. Pharmacol. 1994;46:1217–1225. [PubMed] [Google Scholar]
- Madan A, Faiman MD. Characterization of diethyldithiocarbamate methyl ester sulfine as an intermediate in the bioactivation of disulfiram. J. Pharmacol. Exp. Ther. 1995;272:775–780. [PubMed] [Google Scholar]
- Madan A, Parkinson A, Faiman MD. Identification of the human and rat p450 enzymes responsible for the sulfoxidation of s-methyl n, n-diethylthiolcarbamate (detc-me): the terminal step in the bioactivation of disulfiram. Drug Metab. Dispos. 1995;23:1153–1162. [PubMed] [Google Scholar]
- Madan A, Parkinson A, Faiman MD. Identification of the p-450 enzymes responsible for the sulfoxidation and thiono-oxidation of diethyldithiocarbamate methyl ester: role of P-450 enzymes in disulfiram bioactivation. Alcohol Clin. Exp. Res. 1998;22:1212–1219. [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J. Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J. Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AE, Dewey SL. Effects of pharmacological increases in brain GABA levels on cocaine-induced changes in extracellular dopamine. Synapse. 1998;1:60–65. doi: 10.1002/(SICI)1098-2396(199801)28:1<60::AID-SYN7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Nagendra SN, Faiman MD, Davis K, Wu J-Y, Newby X, Schloss JV. Carbamoylation of brain glutamate receptors by a disulfiram metabolite. J. Biol. Chem. 1997;272:24247–24251. doi: 10.1074/jbc.272.39.24247. [DOI] [PubMed] [Google Scholar]
- Pan Y, Gerasimov MR, Kvist T, Wellendorph P, Madsen KK, Pera E, Lee H, Schousboe A, Chebib M, Brauner-Osborne H, Craft CM, Brodie JD, Schiffer WK, Dewey SL, Miller SR, Silverman RB. (1S, 3S)-3-amino-4-difluoromethylenyl-1-cyclopentanoic acid (CPP-115), a potent γ-Aminobutyric acid aminotransferase inactivator for the treatment of cocaine addiction. J. Med. Chem. 2012;55:357–366. doi: 10.1021/jm201231w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. second ed. San Diego, California: Academic Press; 1986. [Google Scholar]
- Petrakis IL, Carroll KM, Nich C, Gordon LT, McCance-Katz EF, Frankforter T, Rounsaville BJ. Disulfiram treatment for cocaine dependence in methadone-maintained opioid addicts. Addiction. 2000;95:219–228. doi: 10.1046/j.1360-0443.2000.9522198.x. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci. Biobehav. Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Potter DW, Tran TB. Apparent rates of glutathione turnover in rat tissues. Toxicol. Appl. Pharmacol. 1993;120:186–192. doi: 10.1006/taap.1993.1102. [DOI] [PubMed] [Google Scholar]
- Preti A. New developments in the pharmacotherapy of cocaine abuse. Addict. Biol. 2007;12:133–151. doi: 10.1111/j.1369-1600.2007.00061.x. [DOI] [PubMed] [Google Scholar]
- Razoux F, Garcia R, Lena I. Ketamine, at a dose that disrupts motor behavior and latent inhibition, enhances prefrontal cortex synaptic efficacy and glutamate release in the nucleus accumbens. Neuropsychopharmacology. 2007;32:719–727. doi: 10.1038/sj.npp.1301057. [DOI] [PubMed] [Google Scholar]
- Reuter J, Raedler T, Rose M, Hand I, Glascher J, Buchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nat. Neurosci. 2005;8:147–148. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- Scappaticci B, Soubeyrand J, Rousseau-Tsangaris M, Desage M, Brazier JL. Determination of sodium diethyldithiocarbamate (Imuthiol) and its s-methyl metabolite by gas chromatography-mass spectrometry. J. Chromatogr. 1990;534:57–66. [PubMed] [Google Scholar]
- Schloss JV. Therapeutic Compositions, in (USPTO), A61K 038/05; A61K 031/ 325, U.S.A. 2007 [Google Scholar]
- Schroeder JP, Cooper DA, Schank JR, Lyle MA, Gaval-Cruz M, Ogbonmwan YE, Pozdeyev N, Freeman KG, Iuvone PM, Edwards GL, Holmes PV, Weinshenker D. Disulfiram attenuates drug-primed reinstatement of cocaine seeking via inhibition of dopamine beta-hydroxylase. Neuropsychopharmacology. 2010;35:2440–2449. doi: 10.1038/npp.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann. N. Y. Acad. Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- Shorter D, Kosten TR. Novel pharmacotherapeutic treatments for cocaine addiction. BMC Med. 2011;9:119–128. doi: 10.1186/1741-7015-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somaini L, Donnin C, Raggi MA, Amore M, Ciccocioppo R, Saracino MA, Kalluppi M, Malagoli M, Gerra ML, Gerra G. Recent pat. cns Drug Discov. 2011;6:146–160. doi: 10.2174/157488911795933893. [DOI] [PubMed] [Google Scholar]
- Song Y, Lunte CE. Comparison of calibration by delivery versus no net flux for quantitative in vivo microdialysis sampling. Anal. Chim. Acta. 1999;379:251–262. [Google Scholar]
- Spanagel R, Kiefer F. Drugs for relapse prevention of alcoholism: ten years of progress. Trends Pharmacol. Sci. 2008;29:109–115. doi: 10.1016/j.tips.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Strömme JH. Effects of diethyldithiocarbamate and disulfiram on glucose metabolism and glutathione content of human erythrocytes. Biochem. Pharmacol. 1963a;12:705–715. doi: 10.1016/0006-2952(63)90046-7. [DOI] [PubMed] [Google Scholar]
- Strömme JH. Inhibition of hexokinase by disulfiram and diethyldithiocarbamate. Biochem. Pharmacol. 1963b;12:157–166. doi: 10.1016/0006-2952(63)90180-1. [DOI] [PubMed] [Google Scholar]
- Taber MT, Baker GB, Fibiger HC. Glutamate receptor agonists decrease extracellular dopamine in the rat nucleus accumbens in vivo. Synapse. 1996;24:165–172. doi: 10.1002/(SICI)1098-2396(199610)24:2<165::AID-SYN8>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Thomson AM, West DC, Lodge D. An n-methylaspartate receptor-mediated synapse in rat cerebral cortex: a site of action of ketamine? Nature. 1985;313:479–481. doi: 10.1038/313479a0. [DOI] [PubMed] [Google Scholar]
- Trudeau L-E. Glutamate co-transmission as an emerging concept in monoamine neuron function. J. Psychiatry Neurosci. 2004;29:296–310. [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Glutamatergic mechanisms in addiction. Mol. Psychiatry. 2003;8:373–382. doi: 10.1038/sj.mp.4001269. [DOI] [PubMed] [Google Scholar]
- Uys JD, LaLumiere RT. Glutamate: the new frontier in pharmacotherapy for cocaine addiction. CNS Neurol. Disord. Drug Targets. 2008;7:482–491. doi: 10.2174/187152708786927868. [DOI] [PubMed] [Google Scholar]
- Vaccari A, Ferraro L, Saba P, Ruiu S, Mocci I, Antonelli T, Tanganelli S. Differential mechanisms in the effects of disulfiram and diethyldithiocarbamate intoxication on striatal release and vesicular transport of glutamate. J. Pharmacol. Exp. Ther. 1998;285:961–967. [PubMed] [Google Scholar]
- Vaccari A, Saba PL, Ruiu S, Collu M, Devoto P. Disulfiram and diethyldithiocarbamate intoxication affects the storage and release of striatal dopamine. Toxicol. Appl. Pharmacol. 1996;139:102–108. doi: 10.1006/taap.1996.0147. [DOI] [PubMed] [Google Scholar]
- Vocci F, Ling W. Medications development: successes and challenges. Pharmacol. Ther. 2005;108:94–108. doi: 10.1016/j.pharmthera.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Yao L, Fan P, Arolfo M, Jiang Z, Olive MF, Zablocki J, Sun H-L, Chu N, Lee J, Kim H-Y, Leung K, Shryock J, Blackburn B, Diamond I. Inhibition of aldehyde dehydrogenase-2-suppresses cocine seeking by generating THP, a cocaine use-dependent inhibitor of dopamine synthesis. Nat. Med. 2010;16:1024–1028. doi: 10.1038/nm.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngren KD, Daly DA, Moghaddam B. Distinct actions of endogenous excitatory amino acids on the outflow of dopamine in the nucleus accumbens. J. Pharmacol. Exp. Ther. 1993;264:289–293. [PubMed] [Google Scholar]
- Yourick JJ, Faiman MD. Comparative aspects of disulfiram and its metabolites in the disulfiram-ethanol reaction in the rat. Biochem. Pharmacol. 1989;38:413–421. doi: 10.1016/0006-2952(89)90380-8. [DOI] [PubMed] [Google Scholar]
- Yourick JJ, Faiman MD. Disulfiram metabolism as a requirement for the inhibition of rat liver mitochondrial low km aldehyde dehydrogenase. Biochem. Pharmacol. 1991;42:1361–1366. doi: 10.1016/0006-2952(91)90446-c. [DOI] [PubMed] [Google Scholar]