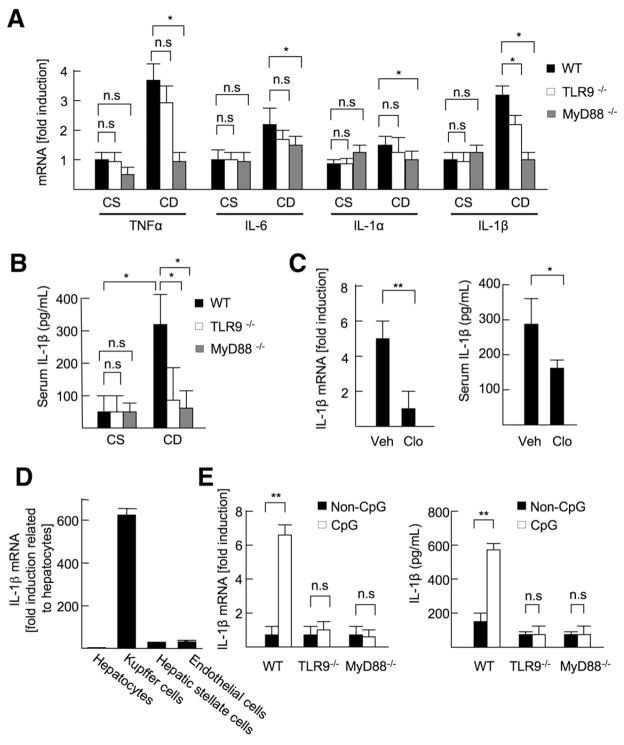
Figure 2.
TLR9 induces IL-1β production in Kupffer cells. (A and B) The livers and sera were harvested from 22-week-old CSAA (CS) or CDAA (CD) diet-fed WT, TLR9−/−, and MyD88−/− mice. (A) Hepatic mRNA levels of inflammatory cytokines were measured by quantitative real-time PCR (qPCR). (B) Serum IL-1β levels were measured by enzyme-linked immunoabsorbent assay (ELISA). (C) WT mice (21 weeks old) fed the CDAA diet were injected control (Veh; n = 6) or clodronate liposome (Clo; n = 6) to deplete Kupffer cells. The livers and sera were harvested 1 week after the liposome injection. Hepatic mRNA and serum protein levels of IL-1β were measured by qPCR and ELISA, respectively. (D) IL-1β mRNA expression in hepatocytes, Kupffer cells, HSCs, and sinusoidal endothelial cells was measured by qPCR. (E) WT, TLR9−/−, and MyD88−/− Kupffer cells were treated with 5 μg/mL CpG-ODN or non–CpG-ODN, and then IL-1β mRNA levels were measured by qPCR. To convert active IL-1β from proIL-1β, Kupffer cells were treated with 2 mmol/L ATP for 30 minutes after 24 hours of incubation with 5 μg/mL CpG-ODN (n = 4, each group), and then secreted IL-1β levels were measured by ELISA. Data represent mean ± SD; *P < .05, **P < .01; n.s., not significant.