Abstract
miR-122 may function as a novel tumor suppressor. Expression of miR-122 could suppress the proliferation of multi-kinds of human cancer cell lines. In this work, expression of miR-122 via adenoviral vector in non-small-cell lung cancer (NSCLC) cells reduces the number of invasion and migration cells. miR-122 attenuates the epithelial–mesenchymal transition process, which mediates cancer cells metastasis in NSCLC cells A549 and H460. The mechanisms data reveals that miR-122 would disrupt the epithelial–mesenchymal transition process by downregulating PI3K/AKT activation via reducing endogenous expression of insulin-like growth factor 1 receptor. These data highlight the detailed roles and potential application of miR-122 in NSCLC cells.
Keywords: microRNA-122, tumor suppressor, metastasis, epithelial-mesenchymal transition, PI3K/AKT signaling pathway
Introduction
MicroRNAs (miRs, miRNAs) are a variety of small noncoding RNAs of 18–25 nucleotides transcribed by RNA Pol III in mammal cells.1 It has been shown that miRs play important roles in proliferation, apoptosis, or metastasis of human cancer cells.1,2 Recently, some miRNAs, including miR-122 or let-7, may function as tumor suppressor and would be used as therapeutic target or strategy for cancer treatment.3 Among these miRs, low level of miR-122 is associated with poor prognosis of hepatocellular carcinoma (HCC).4 Previously, researchers mainly focus on the roles of miR-122 in HCC cells. However, expression of miR-122 can also function as a tumor suppressor and attenuates the proliferation of some other cancer cells, including human uterine cervix cancer line HeLa or large cell lung cancer cell line NCI-H460.4 Jopling4 and Ma et al5 also provide the clues that miR-122 can suppress cancer cells proliferation via posttranscriptional modulation of the endogenous expression of oncogenes Igf1R, cyclin G1 (CCNG1), and Bcl-W by binding to 3′ UTR (untranslated regions) of mRNAs. Results from Ma et al6 show that miR-122 may enhance the sensitivity of non-small-cell lung cancer (NSCLC) cells to antitumor agents. Although miR-122 has been reported to be a tumor suppressor, the studies addressing the detailed roles or potential application of miR-122 are still at an early stage.
NSCLC, which is the most common type of lung cancer, makes up nearly 80% proportion of clinical lung cancer cases.7,8 NSCLC can be further categorized into three subtypes: adenocarcinoma (AC), squamous cell carcinoma, and large cell carcinoma (LCC), and AC accounts for approximately most common subtype of NSCLC diagnosed in patients.9 NSCLC is one of the most fatal human malignancies, and majority of patients suffering from NSCLC are surgically unrespectable due to advanced stage disease at first diagnosish.10 Therefore, there is urgent need to develop novel strategies or to improve the efficacy of traditional therapy.
As a target of miR-122, insulin-like growth factor 1 receptor (IGF1R), a kind of receptor tyrosine kinase, plays a critical role in cancer development and progression.11–13 Activated IGF1R may initiate a signaling cascade axe, the PI3K/AKT signaling pathway.11–13 It has been confirmed that AKT can induce epithelial–mesenchymal transition (EMT), an important process toward cancer cell metastasis.11–13 In the current study, we show that expression of miR-122 via adenoviral vector significantly reduced the proliferation, metastasis, and EMT process of NSCLC cell lines A549 and H460. miR-122 significantly reduces the expression of its targets, IGF1R or BCL-W (B-cell lymphoma W), and the activation of PI3K/AKT signaling pathway.
Materials and methods
Ethics statement
Our study aims to declare the potential function and mechanisms of miR-122 in lung cancer treatment. The work did not include any materials from clinical specimens, or the clinical trial or methods. No ethics statement was required from the institutional review board for the use of human cell lines.
Cell culture and proliferation analysis
WI-38, human embryonic lung cell line, A549 (AC) and H460 (LCC), HCC cell lines HepG2, MHCC-97H, MHCC-97L, and non-tumor hepatocyte L-02 cell line were obtained from cell resources center of Chinese Academy of Medical Sciences and Peking Union Medical College or Type Culture Collection of the Chinese Academy of Sciences (Shanghai) in People’s Republic of China. Cells were cultured in complete Dulbecco’s Modified Eagle’s Medium (DMEM) (Invitrogen, Carlsbad, CA, USA) in a sterile incubator maintained at 37°C with 5% CO2. For measuring proliferation ability, A549 or H460 cells were harvested and analyzed by MTT-assays.14 Briefly, cells were seeded in 96-well cell culture plates. After incubating for 1, 2, 3, and 4 days, cells were harvested and analyzed by MTT-assays. The absorbance of the inhibition rate was measured using a multifunctional micro-plate reader at 490 nm. The MTT-cell growth assays were performed for three independent times.
Adenovirus vector preparation
The adenovirus vector Ad-miR122 was constructed following the methods described by Niu et al.7 The sequence of miR-122 that was cloned into pShuttle-CMV vector was generated through annealing of two oligo-DNA fragments with sticky end/cohesive terminus at sites of BamH I and Hind III: forward sequence, 5′-GATCCGCCTTAGCAGAGCTGTGGAGTGTGACAATGGTGTTTGTGTCTAAACTATCAA ACG-3′; reverse sequence, 5′-AGCTTAAAAAAGGCCTAGCAGTAGCTATTTAGTGTGATAATGGCGTTTGATAGTTTAGACA-3′.
RNA isolation and real-time reverse transcription polymerase chain reaction
The miRNA examination kits of miR-122 were obtained from Applied Biosystems Company (Foster City, CA, USA). RNA isolation and real-time reverse transcription polymerase chain reaction (RT-PCR) were performed using the ABI PRISM 7500 Sequence Detection System (Applied Biosystems) according to standard procedures from the manufacturer’s protocol. The housekeeping protein β-actin was chosen as loading control, and relative expression levels were normalized to the expression of human β-actin mRNA. Relative expression of miR-122 was calculated by using average of the control group as a calibrator. The primers of β-actin were: forward primer, 5′-CTCCATCCTGGCCTCGCTGT-3′; reverse primer, 5′-GCTGTCACCTTCACCGTTCC-3′.
Transwell assay
A549 or H460 cells were analyzed by transwell analysis. The transwell analysis was performed in 24-well plates using transwell chambers (Corning Incorporated, Corning, NY, USA) fitted with a polyethylene terephthalate filter membrane with 8 μm pores. For invasion test, the membrane undersurface was coated with 30 μL extracellular matrix gel (Sigma-Aldrich Co., St Louis, MO, USA) mixed with RPMI 1640 serum-free medium in 1:5 dilution for 4 hours at 37°C. The top chambers were filled with 0.2 mL of cells (5×105 cells/mL) in serum-free medium, and the bottom chambers were filled with 0.25 mL of RPMI 1640 medium containing 10% fetal bovine serum. The cells were incubated in the transwell chambers at 37°C in 5% CO2 for 12 hours (invasion) or 4 hours (migration). The relative invasion–migration cells were measured following the methods descripted by Egloff et al15 or Chen et al.16
Antibodies and Western blot
Antibodies against insulin-like growth factor 1 receptor, N-cadherin, E-cadherin, Vimentin, phospho-AKT, phospho-P110 (PI3K catalytic subunit), AKT, P110, and glyceraldehyde phosphate dehydrogenase were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Secondary antibodies conjugated with the horseradish peroxidase were from Sigma Company. The Western blot analysis was performed following methods descripted by Zhang et al.17 The primary antibodies against insulin-like growth factor receptor (1:1,000), N-cadherin (1:2,000), E-cadherin (1:1,000), Vimentin (1:1,000), phospho-AKT (1:500), phospho-P110 (1:500), AKT (1:1,000), P110 (1:1,000), glyceraldehyde phosphate dehydrogenase (1:5,000), and horseradish peroxidase-conjugated secondary antibodies (1:5,000) were diluted in tris-buffered saline and Tween 20 containing 5% bovine serum albumin.
Luciferase assay
The luciferase kits were obtained from Promega Corporation, Fitchburg, WI, USA. The experiments were performed following the protocol from the manufacturer. The luciferase reporter of Smad4 binding site (SBE-Luc) was from Dr Fan Feng in Center of Therapeutic Research for Hepatocellular Carcinoma, Beijing 302nd Hospital, Beijing, People’s Republic of China.18 Briefly, A549 or H460 cells were seeded in 24-well plates (Corning Incorporated) containing RPMI 1640 medium supplemented with 10% fetal bovine serum. Cells were co-transfected with the luciferase reporters and then harvested after culture for 24 hours. The luciferase and β-galactosidase activities were then examined. The luciferase assays were performed three times with similar results.
Statistical analysis
The relative expression level of results from real-time RT-PCR or Western blotting analyzed by Alpha Innotech (San Leandro, CA, USA) was calculated as follows: (indicated group expression level/loading control expression level)/(control group expression level/loading control expression level).19,20 Statistical significance was determined using SPSS 9.0 (SPSS Inc., Chicago, IL, USA) statistical software using Bonferroni’s correction with or without two-way analysis of variance.
Results
miR-122 expresses in HCC and hepatocyte non-tumor cells but not in NSCLC cells
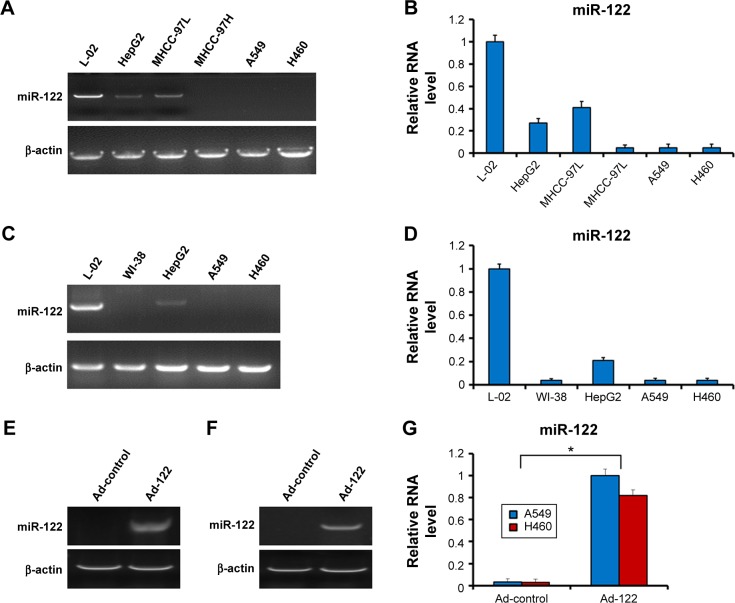
The baseline expression of endogenous miR-122 in cells was examined by real-time RT-PCR. As shown in Figure 1A and B, endogenous expression of miR-122 was detected in L-02, HepG2, and MHCC-97L cells but not in MHCC-97H, NSCLC A549, and H460 cells. Furthermore, to confirm the expression of miR-122, human embryonic lung cell line WI-38 was also used. As shown in Figure 1C and D, no endogenous expression of miR-122 was detected. And the expression of miR-122 was significantly downregulated in HepG2 and A549 or H460 (Figure 1C and D). This result confirmed the fact that miR-122 is highly enriched in the liver tissue and downregulated in tumor tissues. Therefore, we chose A549 cell (AC) and H460 (LCC) representing the most common types of NSCLC.
Figure 1.
miR-122 expression is detected in hepatocyte cells but not in NSCLC cells, A549 or H460.
Notes: Total RNA, which was extracted from the indicated cell lines, was analyzed by real-time RT-PCR, and β-actin was chosen as loading control. (A and C) The endogenous level of miR-122 or β-actin was shown as DNA gel electrophoretic bands. (B and D) Relative RNA level was shown as mean ± SD from triplicate measurements with similar results. The expression of miR-122 in NSCLC via ad vector is shown as photograph (E and F) or relative RNA level (D and G). *P<0.05.
Abbreviations: NSCLC, non-small-cell lung cancer; RT-PCR, reverse transcription polymerase chain reaction.
miR-122 suppresses proliferation of A549 and H460 cells
Next, the effect of miR-122 on proliferation of A549 and H460 cells was investigated. Expression of miR-122 in A549 (Figures 1E and G and 2A) or H460 (Figures 1F and G and 2B) cells via Ad-122 resulted in a reduction proliferation than those infected with Ad-control. There was no significant difference of proliferation between cells infected with Ad-control and the parental cells (Figure 2). These data showed that miR-122 suppressed A549 and H460 cells proliferation.
Figure 2.
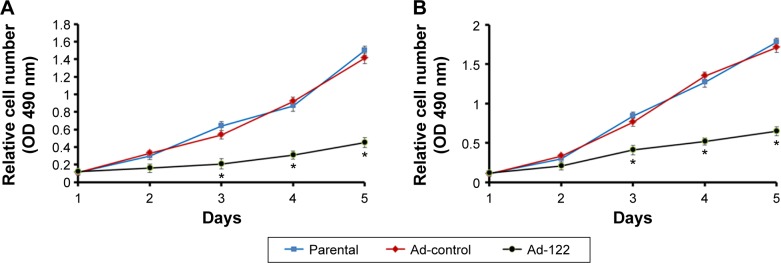
Expression of miR-122 inhibits the proliferation of A549 or H460 cells.
Notes: A549 (A) or H460 (B) cells which were infected with Ad-control, Ad-122, or parental cells were analyzed by MTT-assays. All values were shown as mean ± standard deviation from triplicate experiment with similar results. *P<0.05 versus Ad-control or Ad-122.
Abbreviation: MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
miR-122 reduces the metastasis of A549 and H460 cells with decreased EMT process
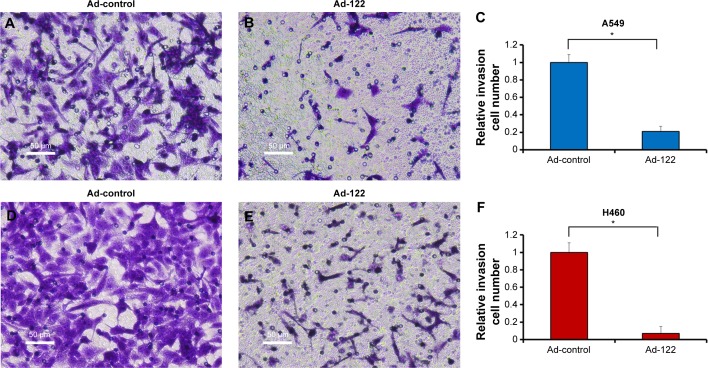
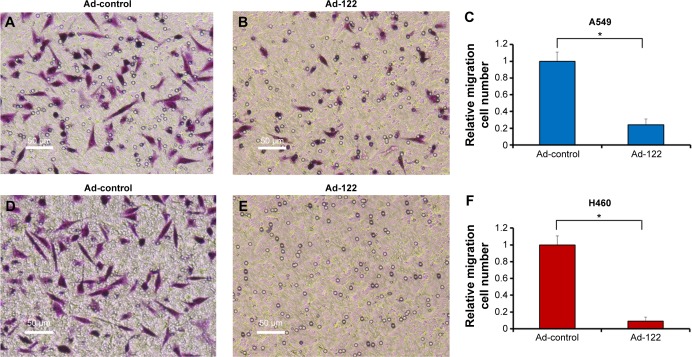
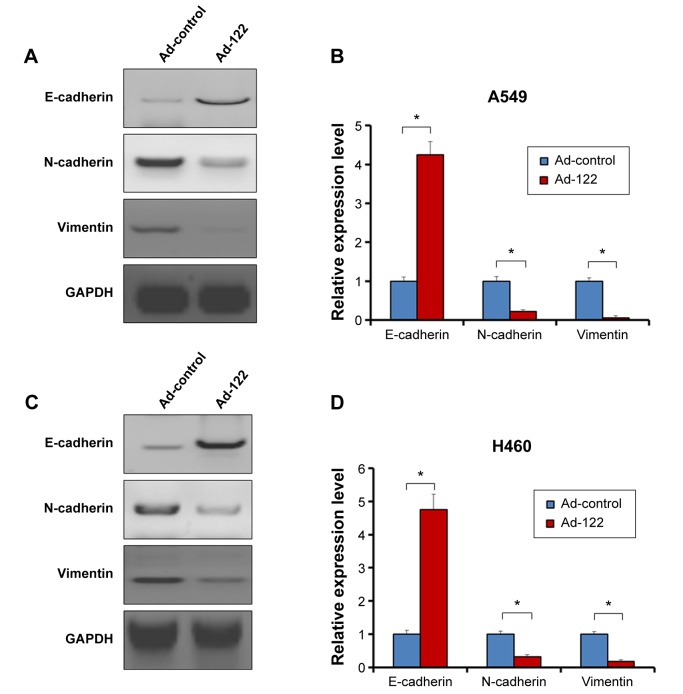
Invasion and migration (metastasis) are main features of human cancer malignancies. Results from transwell assays revealed that miR-122 significantly inhibited the invasion of A549 and H460 cells (Figure 3). Similar results were obtained in migration analysis (Figure 4). Furthermore, miR-122 also disrupted the EMT process of A549 and H460 cells (Figure 5). As shown in Figure 5, miR-122 increased the expression of the epithelial marker E-cadherin and reduced that of N-cadherin and Vimentin, two mesenchymal markers. The results indicated that miR-122 would disrupt NSCLC cells metastasis via EMT process.
Figure 3.
Expression of miR-122 inhibits invasion of A549 or H460 cells.
Notes: A549 (A–C) and H460 (D–F) cells which were infected with Ad-control or Ad-122 were analyzed by transwell assays. The results were shown as typical photograph (A, B, D, and E) or relative invasion cell number (mean ± standard deviation) from triplicate experiment with similar results (C and F). *P<0.05 versus Ad-control or Ad-122.
Figure 4.
Expression of miR-122 inhibits migration of A549 or H460 cells.
Notes: A549 (A–C) and H460 (D–F) cells which were infected with Ad-control or Ad-122 were analyzed by transwell assay. The results were shown as typical photograph (A, B, D, and E) or relative invasion cell number (mean ± standard deviation) from triplicate experiment with similar results (C and F). *P<0.05 versus Ad-control or Ad-122.
Figure 5.
Expression of miR-122 modulates the expression of EMT markers.
Notes: A549 (A and B) or H460 (C and D) cells, which were infected with Ad-control or Ad-122, were harvested and analyzed by Western blot assays. The protein level of epithelial marker E-cadherin and of N-cadherin or Vimentin, two mesenchymal markers, was detected by antibodies. Relative expression level was shown as mean ± SD from triplicate experiment with similar results (B and D). *P<0.05 versus Ad-control or Ad-122.
Abbreviations: GAPDH, glyceraldehyde phosphate dehydrogenase; SD, standard deviation.
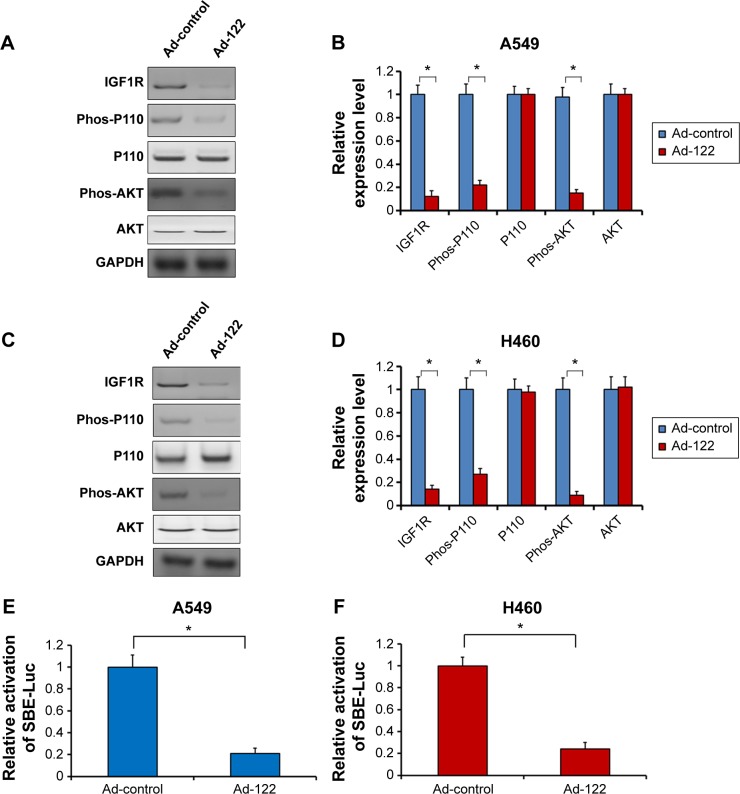
miR-122 reduces the activation of PI3K/AKT signaling pathway in A549 and H460 cells
To further investigate how miR-122 regulates EMT markers, the activation of PI3K/AKT signaling pathway was examined. Expression of miR-122 significantly downregulated the protein level of IGF1R and the phosphorylation of PI3K catalytic subunit P110 or AKT (Figure 6A–D). Next, to confirm the specificity of miR-122 activity, the protein level of PI3K catalytic subunit P110 and AKT was also examined. Expression of miR-122 did not modulate the endogenous level of P110 and AKT (Figure 6A–D). These all suggested that miR-122 may attenuate the EMT process via PI3K/AKT signaling pathway.
Figure 6.
Expression of miR-122 reduces the activation of IGF1R/PI3K/AKT signaling pathway.
Notes: A549 (A and B) or H460 (C and D) cells, which were infected with Ad-control or Ad-122, were harvested and analyzed by Western blot assays. The protein level of IGF1R, PI3K catalytic subunit P110, phospho-P110, AKT, and phospho-AKT was detected by antibodies. Relative expression level was shown as mean ± SD from triplicate experiment with similar results (B and D). A549 (E) or H460 (F) cells, which were infected with Ad-control or Ad-122, were co-transfected with SBE-Luc reporters. Cells were harvested and analyzed for luciferase analysis. The relative luciferase activity was shown as mean ± SD from triplicate experiment with similar results (E and F). *P<0.05 versus Ad-control or Ad-122.
Abbreviations: IGF1R, insulin-like growth factor 1 receptor; GAPDH, glyceraldehyde phosphate dehydrogenase; SD, standard deviation; PI3K, phosphatidylinositol 3 kinase.
Moreover, based on the evidence that transforming growth factor beta (TGF-β)/Smads signaling pathway is also participated in EMT process, the effect of miR-122 on TGF-β/Smads signaling pathway was detected. A549 or H460 cells were co-transfected with Ad-control, Ad-122, or SBE-Luc (Smad4 biding site), a luciferase reporter reflecting the activation of TGF-β/Smads signaling pathway. As shown in Figure 6E and F, miR-122 significantly reduced the activity of SBE-Luc in A549 or H460 cells. These data indicated that miR-122 may also modulate the activation of TGF-β/Smads signaling pathway.
Discussion
Recently, miR-122 has been implicated as a tumor suppressor. Increasing evidences provided the application of miR-122 in tumor treatment. Ma et al5 reported that overexpression of miR-122 reduces the proliferation of HCC, cervix cancer, and large cell lung cancer cell line NCI-H460. Other studies also showed that expression of miR-122 could sensitize HCC or NSCLC cells to antitumor agents, including gemcitabine (Ma et al6), sorafenib (Bai et al21), or doxorubicin (Fornari et al22). Although the roles of miR-122 has been declared and understood in HCC, the studies addressing the potential application of miR-122 as a tumor suppressor are still at an early stage. In this work, our results reveal the critical function of miR-122 in NSCLC. First, there is no endogenous expression of miR-122 in NSCLC cells, suggesting the specific and significant activity of miR-122 by targeting to its targets IGF1R or BCL-W, which mediates the proliferation or anti-apoptosis in regulation of NSCLC cell growth. Second, miR-122 attenuates the metastasis of A549 and H460 cells with decreased EMT. Mechanistically, miR-122 may disrupt metastasis or EMT process through IGF1R/PI3K/AKT cascade. In addition, miR-122 also decreases the activation of TGF-β/Smads signaling pathway, which also participates in EMT process. These findings indicated that miR-122 may function as a tumor suppressor and a potential target for NSCLC therapy.
The abilities of metastasis are main features of metastatic malignancies, which highly related to the development, progression, or prognosis of human cancers. EMT plays a critical role in cancer cell migration and invasion.23 Receptor tyrosine kinases, such as IGF1R may promote the metastasis of cancer cells and initiates PI3K/AKT cascade.17,24 Lamouille and Derynck,11 Pon et al,12 and Smith et al13 revealed that PI3K/AKT signaling pathway could induce the EMT process and in turn promote the metastasis of cancer cells. Activation of AKT is also responsible for EMT markers E-cadherin and N-cadherin.23 In this work, expression of miR-122 reduces the metastasis and of A549 and H460 cells with decreased EMT. miR-122 significantly downregulates the endogenous level of IGF1R, and the PI3K P110/AKT phosphorylation but not protein level. Since IGF1R is a target of miR-122, our data illustrates that expression of miR-122 would disrupt EMT via PI3K/AKT signaling pathway. Besides, TGF-β/Smads cascade is also involved in EMT process.25 The Smads protein family, including SMAD2, SMAD3, and SMAD4, mediate transcription of targeted genes in response to TGF-β.26 In human cancer cells, TGF-β binds to type I and type II transmembrane receptors, which form a heteromeric receptor complex, and in turn phosphorylates/activates the cytoplasmic Smad2/3 proteins at their C-terminal SSXS motif.26 Next, activated Smad2/3 interacts with Smad4 to form a complex, which subsequently translocates to nucleus and mediates the transcription of target genes via binding to Smad4 binding site located the promoter sequences of its target genes.26 In this work, miR-122 reduces the activity of SBE-Luc, which reflects the activation of TGF-β/Smads. Li et al25 and Yu et al27 indicate that PI3K could induce the phosphorylation of Smad2/3. Collaborated with our results, miR-122 would modulate the activation of TGF-β/Smads signaling pathway via IGF1R/PI3K pathway.
Interestingly, results from the study by Wang et al28 show that miR-122 would trigger the mesenchymal–epithelial transition, a reverse process of EMT, and suppress HCC cells motility and invasion by targeting Rho A. This result supports our findings from another side. Moreover, Eliasz et al10 also shows that activated IGF1R pathway stimulates survival of lung AC cells via Notch-1 signaling pathway. Saad et al29 found that Notch-1 activation would be involved in EMT process. It is valuable to further delineate the effect of miR-122 on Notch-1 pathway in NSCLC regulation.
Conclusion
In summary, we demonstrated that expression of miR-122 via adenoviral vector exerted strong antitumor activity and effectively disrupted metastasis of NSCLC cells. miR-122 also attenuated the EMT process of NSCLC cells. These all indicated the application of miR-122 as a novel target of NSCLC therapy.
Acknowledgments
This work was supported by Beijing Municipal Natural Science Foundation (7122135).
Footnotes
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in either drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cullen BR. Viral and cellular messenger RNA targets of viral microRNAs. Nature. 2009;457(7228):421–425. doi: 10.1038/nature07757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qian B, Nag SA, Su Y, et al. miRNAs in cancer prevention and treatment and as molecular targets for natural product anticancer agents. Curr Cancer Drug Targets. 2013;13(5):519–541. doi: 10.2174/15680096113139990031. [DOI] [PubMed] [Google Scholar]
- 3.Takamizawa J, Konishi H, Yanagisawa K, et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64(11):3753–3756. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 4.Jopling C. Liver-specific microRNA-122: biogenesis and function. RNA Biol. 2012;9(2):137–142. doi: 10.4161/rna.18827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma L, Liu J, Shen J, et al. Expression of miR-122 mediated by adenoviral vector induces apoptosis and cell cycle arrest of cancer cells. Cancer Biol Ther. 2010;9(7):554–561. doi: 10.4161/cbt.9.7.11267. [DOI] [PubMed] [Google Scholar]
- 6.Ma DB, Feng F, Zhang F, et al. MicroRNA122 enhances the cytotoxic activity of gemcitabine on A549 cells. Acad J Chin PLA Med Sch. 2014;35:1160–1164. [Google Scholar]
- 7.Niu C, Liang CL, Guo JT, et al. Downregulation and growth inhibitory role of FHL1 in lung cancer. Int J Cancer. 2011;130(11):2549–2556. doi: 10.1002/ijc.26259. [DOI] [PubMed] [Google Scholar]
- 8.Herbst RS, Heymach JV, Lippman SM. Molecular origins of cancer: lung cancer. N Engl J Med. 2008;359:1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webb JD, Simon MC. Novel insights into the molecular origins and treatment of lung cancer. Cell Cycle. 2010;9(20):4098–4105. doi: 10.4161/cc.9.20.13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eliasz S, Liang S, Chen Y, et al. Notch-1 stimulates survival of lung adenocarcinoma cells during hypoxia by activating the IGF-1R pathway. Oncogene. 2010;29(17):2488–2498. doi: 10.1038/onc.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamouille S, Derynck R. Cell size and invasion in TGF-beta-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J Cell Biol. 2007;178(3):437–451. doi: 10.1083/jcb.200611146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pon YL, Zhou HY, Cheung AN, Ngan HY, Wong AS. p70S6 kinase promotes epithelial to mesenchymal transition through snail induction in ovarian cancer cells. Cancer Res. 2009;68(16):6524–6532. doi: 10.1158/0008-5472.CAN-07-6302. [DOI] [PubMed] [Google Scholar]
- 13.Smith AP, Verrecchia A, Fagà G, et al. Apositive role for Myc in TGFb-induced Snail transcription and epithelial-to-mesenchymal transition. Oncogene. 2009;28(3):422–430. doi: 10.1038/onc.2008.395. [DOI] [PubMed] [Google Scholar]
- 14.Li MY, Zhu M, Feng F, et al. Long interspersed nucleotide acid element-1 ORF-1 protein promotes proliferation and invasion of human colorectal cancer LoVo cells through enhancing ETS-1 activity. Genet Mol Res. 2014;13(3):6981–6994. doi: 10.4238/2014.April.14.13. [DOI] [PubMed] [Google Scholar]
- 15.Egloff AM, Rothstein ME, Seethala R, et al. Cross-talk between estro-gen receptor and epidermal growth factor receptor in head and neck squamous cell carcinoma. Clin Cancer Res. 2009;15(21):6529–6540. doi: 10.1158/1078-0432.CCR-09-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Feng F, Gao XD, et al. MiRNA153 reduces effects of chemotherapeutic agents or small molecular kinase inhibitor in HCC cells. Curr Cancer Drug Targets. 2015;15(3):176–187. doi: 10.2174/1568009615666150225122635. [DOI] [PubMed] [Google Scholar]
- 17.Zhang F, Feng F, Yang P, et al. Four-and-a-half-LIM protein 1 down-regulates estrogen receptor α activity through repression of AKT phosphorylation in human breast cancer cell. Int J Biochem cell Biol. 2012;44(2):320–326. doi: 10.1016/j.biocel.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Feng F, Lu YY, Zhang F, et al. Long interspersed nuclear element ORF-1 protein promotes proliferation and resistance to chemotherapy in hepatocellular carcinoma. World J Gastroenterol. 2013;19(7):1068–1078. doi: 10.3748/wjg.v19.i7.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu Y, Feng F, Yang Y, et al. LINE-1 ORF-1p functions as a novel androgen receptor co-activator and promotes the growth of human prostatic carcinoma cells. Cell Signal. 2013;25(2):479–489. doi: 10.1016/j.cellsig.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Yang Q, Feng F, Zhang F, et al. LINE-1 ORF-1p functions as a novel HGF/ETS-1 signaling pathway co-activator and promotes the growth of MDAMB-231 cell. Cell Signal. 2013;25(12):2652–2660. doi: 10.1016/j.cellsig.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 21.Bai S, Nasser MW, Wang B, et al. MicroRNA-122 inhibits tumorigenic properties of hepatocellular carcinoma cells and sensitizes these cells to sorafenib. J Biol Chem. 2009;284(46):32015–32027. doi: 10.1074/jbc.M109.016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fornari F, Gramantieri L, Giovannini C, et al. MiR-122/cyclin G1 interaction modulates p53 activity and affects doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 2009;69(14):5761–5767. doi: 10.1158/0008-5472.CAN-08-4797. [DOI] [PubMed] [Google Scholar]
- 23.Feng YY, Xu XJ, Zhang YJ, et al. HPIP is upregulated in colorectal cancer and regulates colorectal cancer cell proliferation, apoptosis and invasion. Sci Rep. 2015;5:9429. doi: 10.1038/srep09429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng Q, Xia C, Fang J, et al. Role of PI3K and AKT specific isoforms in ovarian cancer cell migration, invasion and proliferation through the p70S6K1 pathway. Cell Signal. 2006;18(12):2262–2271. doi: 10.1016/j.cellsig.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 25.Li CH, Tang LP, Zhao LJ, et al. OPCML is frequently methylated in human colorectal cancer and its restored expression reverses EMT via downregulation of smad signaling. Am J Cancer Res. 2015;5(5):1635–1648. [PMC free article] [PubMed] [Google Scholar]
- 26.Ding L, Wang Z, Yan J, et al. Human four-and-a-half LIM family members suppress tumor cell growth through a TGF-β-like signaling pathway. J Clin Invest. 2009;119(2):349–361. doi: 10.1172/JCI35930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu JS, Ramasamy TS, Murphy N, et al. PI3K/mTORC2 regulates TGF-b/activin signaling by modulating Smad2/3 activity via linker phosphorylation. Nat Commun. 2015;6:7212. doi: 10.1038/ncomms8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang SC, Lin XL, Li J, et al. MicroRNA-122 triggers mesenchymal-epithelial transition and suppresses hepatocellular carcinoma cell motility and invasion by targeting RhoA. PLoS One. 2014;9(7):e101330. doi: 10.1371/journal.pone.0101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saad S, Stanners SR, Yong R, Tang O, Pollock CA. Notch mediated epithelial to mesenchymal transformation is associated with increased expression of the Snail transcription factor. Int J Biochem Cell Biol. 2010;42(7):1115–1122. doi: 10.1016/j.biocel.2010.03.016. [DOI] [PubMed] [Google Scholar]