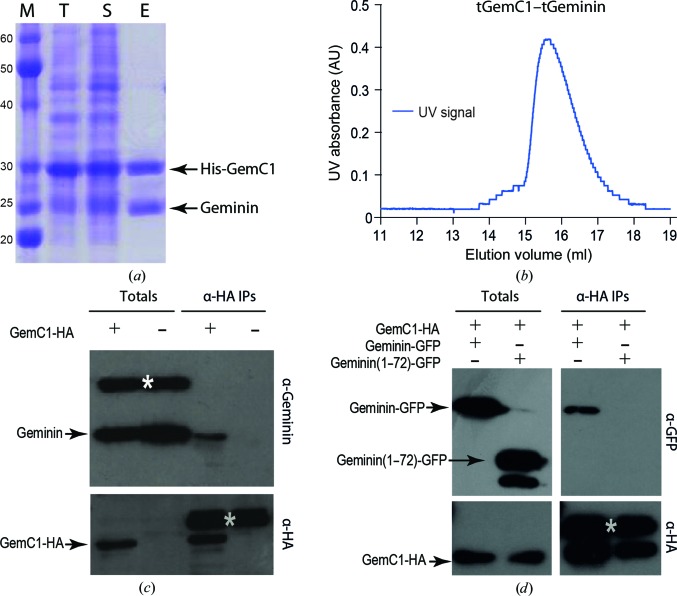
Figure 4.
GemC1 heterodimerizes with Geminin. (a) Co-expression of His-tagged dGemC1 (29–240) and untagged dGeminin (29–209) in E. coli. GemC1 is the best overexpressed protein both in the total cell lysate (lane T) and the supernatant (lane S). However, purification by Ni2+ affinity results only in an approximately stoichiometric GemC1–Geminin complex (lane E). Lane M contains molecular-weight markers (labelled in kDa). (b) Size-exclusion chromatography of the tGemC1–tGeminin heterodimer showing the normalized UV280 nm elution profile (blue line). (c) HA-tagged GemC1 was overexpressed in U2OS cells and was able to co-precipitate endogenous Geminin, suggesting that the two proteins also interact in human cells; in the lower panel the grey star marks the large chain of the IgGs present in the anti-HA immunoprecipitates; the white star in the upper panel marks a band cross-reacting with the Geminin antibody. (d) HA-tagged GemC1 was overexpressed in U2OS cells in the presence of either GFP-tagged Geminin or a construct of Geminin lacking the coiled-coil domain, Geminin(1–72), indicating that the Geminin coiled coil is necessary for interaction with GemC1 in U2OS cells; the grey star marks the large chain of the IgGs present in the anti-HA; the unlabelled band below Geminin(1–72)-GFP is most likely to be a degradation product.