Figure 1.
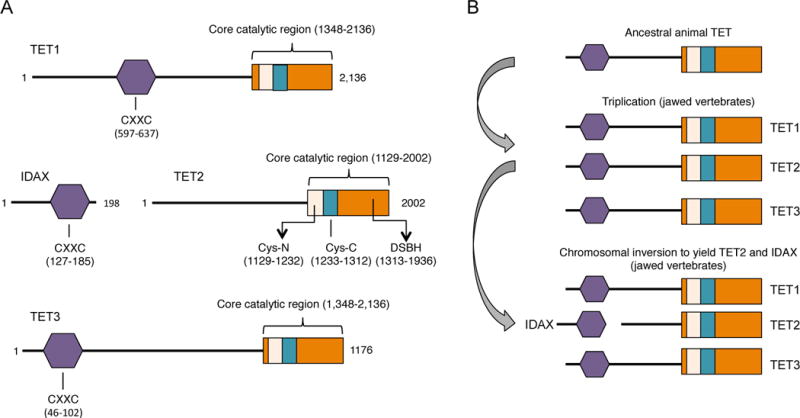
A, Schematic representation of the predicted functional domains of mammalian TET proteins. Depicted is the CXXC domain that is present in TET1 and TET3, the C-terminal catalytic domain that contains a cysteine-rich (Cys-rich) region and the double-stranded β-helix (DSBH) region that are common features in all three members of the TET family. The crystal structure of human TET2 reveals that the Cys-rich region is divided into N- and C-terminal regions that flank the DSBH domain (Hu et al. 2013). Also depicted is IDAX, a CXXC-domain protein that was part of TET2 prior to chromosomal inversion ((Pastor et al. 2013), see B). The numbers indicate aminoacids and they correspond to human TET proteins. B, Evolutionary history of TET proteins. The original gene encoding TET underwent triplication in jawed vertebrates, giving rise to the three members of the TET family. A subsequent chromosomal inversion resulted in the separation of the TET2 catalytic domain from the CXXC domain, which became a separate gene, IDAX/CXXC4.
