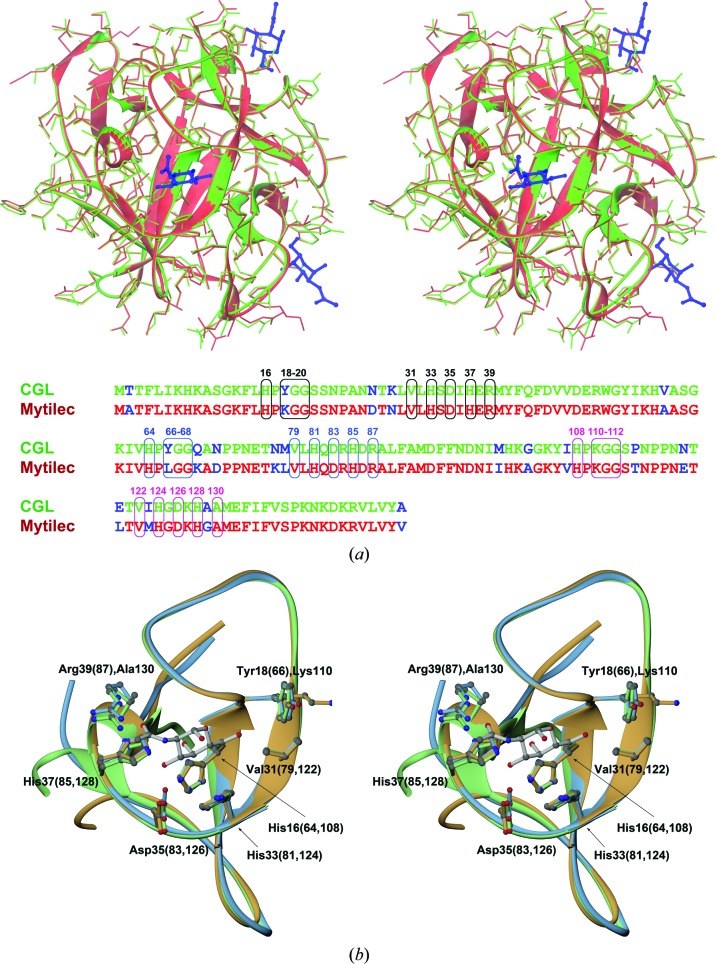
Figure 5.
(a) A stereoimage showing the superposition of monomers of CGL (shown in green) and MytiLec (shown in red; PDB entry 3wmv) in an orientation displaying one of the carbohydrate-binding sites of MytiLec at the front. In addition to the main chains, shown as ribbons, all side chains are depicted in stick representation. Three molecules of α-GalNAc identified in the structure of MytiLec are also shown and are colored dark blue. At the bottom of this figure the amino-acid sequences of both lectins are aligned, which differ at 19 positions (shown in blue). Residues forming the α-GalNAc-binding sites in MytiLec and their equivalents in CGL are shown in boxes and labeled using different colors for each site. (b) A stereoimage of the three aligned CGL subdomains, colored according to the scheme introduced in Fig. 4 ▸. Fragments are shown from the view of a putative carbohydrate-binding site together with a model of the α-GalNAc molecule, a likely ligand of CGL (see text). Side chains of amino acids interacting with α-GalNAc are also shown for all three repeats and are labeled according to their positions in the CGL sequence.