Abstract
Historically, fat was considered detrimental to health and lifespan. However, lipidomics, the quantification of all lipid molecules in a biological sample, and genetic studies in model organisms are revealing specific fats that may promote longevity. These emerging findings provide insight into the complex relationship between lipids and longevity.
Keywords: aging, lipidomics, lipid signaling
Lipids and lifespan
Lipids are essential components of biological membranes, energy sources, and signaling molecules. Lipid signals influence fat synthesis, storage, or catabolism, creating intricate metabolic networks that allow organisms to respond to nutrient availability, energy demands, or otherwise adapt to changing environments [1]. Storage lipids (triglycerides) and circulating lipid-protein complexes (lipoprotein particles) have previously been linked to diseases of aging [2], and obesity limits longevity [3]. But can lipids beneficially influence lifespan? Lipid profiles of longlived humans and model organisms and genetic studies of lipid metabolism suggest that this is the case.
Characterizing lipids presents unique challenges, but new technologies facilitate quantitative detection of diverse lipids in human samples and model organisms typically used in aging studies (Box 1). The nematode C. elegans has emerged as a powerful model for lipid studies due to the detailed characterization of metabolic pathways and ease of genetic manipulation [4,5]. Despite differences in how lipids are stored and synthesized between worms and mammals [4], some lipid profiles associated with longevity may be conserved, and C. elegans has uncovered molecular mechanisms relating lipids to lifespan that could be explored in mammals.
BOX 1: Lipid profiling challenges and technologies.
Lipids exist in a staggering array of sizes, biophysical properties, and relative abundance. An organism’s lipid profile is determined by the combined influences of dietary lipids, de novo lipogenesis, and the hundreds of enzymes that modulate the length and desaturation of fatty acid (FA) chains and their incorporation into more complex lipid molecules. For example, desaturases convert saturated FA chains to monounsaturated (MUFA, one carbon-carbon double bond) and polyunsaturated (PUFA, two or more double bonds) FAs (Figure 1). Lipases liberate FAs from lipid molecules to serve as energy sources or signals or to facilitate transport to other tissues [10]. Current methods utilizing liquid chromatography mass spectrometry (LC-MS) can detect hundreds of unique lipid species, including novel lipid molecules [13]. Although it is still not possible to detect all types of lipid molecules in a sample, LC-MS based methods combined with sophisticated software to aid in lipid identification can detect hundreds of distinct lipids. Other methods that degrade lipids into FA chain components can detect differences in FA structure at high resolution, revealing trends in lipid profiles [1,13]. Analysis of metabolites, without specifically targeting lipids, can also identify some lipid molecules [9].
Lipid profiles associated with longevity
Several lipidomic studies in humans have revealed trends in lipid composition associated with long life. Total lipids extracted from plasma or isolated from erythrocyte membranes of children of long-lived individuals (nonagenarians or centenarians) contain a higher ratio of monounsaturated (MUFA) to polyunsaturated (PUFA) fatty acid chains, relative to matched controls [2]. An increased MUFA:PUFA ratio may influence lifespan by reducing oxidative stress and damage. All lipids can be oxidized by free radicals, but PUFAs are most susceptible to oxidation [2,6]. Because oxidation of fatty acids (FAs) propagates further free radical production, high levels of PUFAs could increase oxidative damage on a much larger scale [6].
Several long-lived mutants in C. elegans, including mutants with reduced insulin-like signaling and dietary restriction mimics, also exhibited modestly increased MUFA:PUFA ratios when analyzed for general fatty acid chain composition, with the longest-lived mutants having the highest MUFA:PUFA ratios [6]. These worms, and worms lacking a germline (which are also long-lived), express higher levels of enzymes that convert saturated FAs to MUFAs (Δ9 desaturases) [3,6] (Figure 1). Additionally, knockdown of Δ5 desaturase, which produces highly unsaturated FAs, appears to promote oxidative stress resistance and lifespan extension in wild-type worms [6]. These findings support a link between PUFA synthesis, oxidative damage, and aging. In mammals, pathways that influence longevity, including insulin and growth factor signaling, regulate Δ9 desaturase expression [3], suggesting that these enzymes may be important targets of other modes of lifespan extension. Future studies are needed to explore the contribution of FA desaturases and their products to longevity in worms and mammals, especially in the context of long-lived mutants that are known to have numerous other cellular effects.
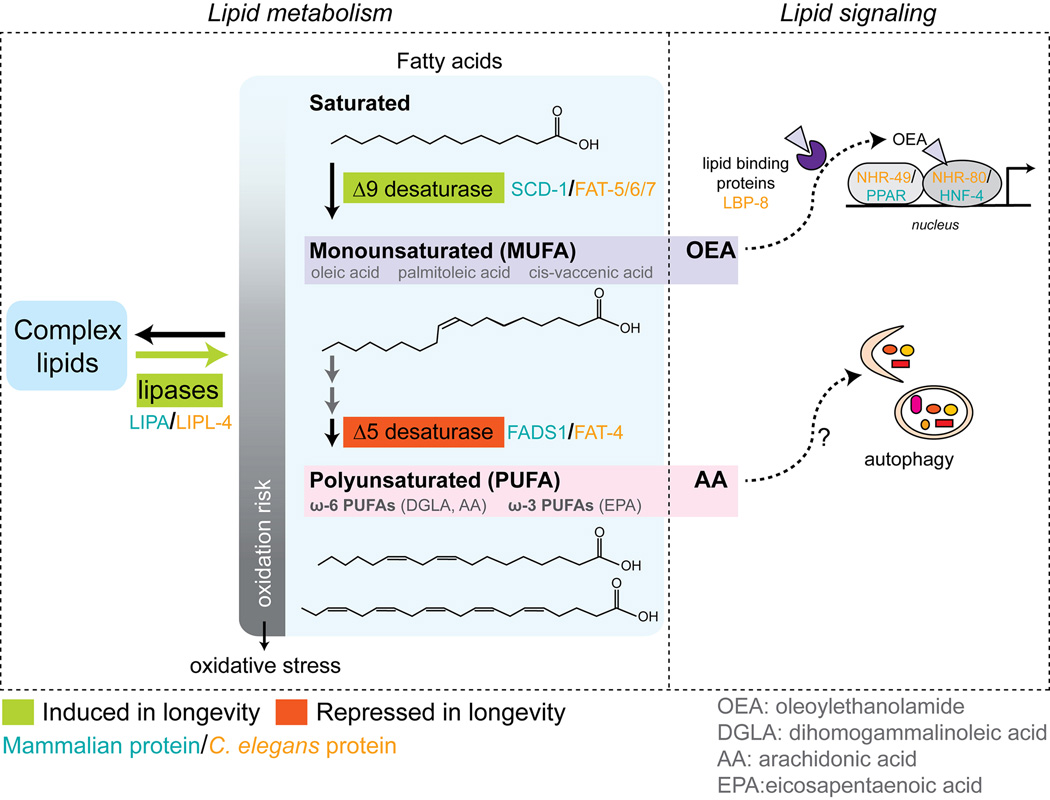
FIGURE 1. Lipid metabolic pathways, profiles, and signals implicated in longevity.
Free fatty acid (FA) chains can be liberated from complex fats by the activities of lipases. Several lipases are elevated under conditions that extend lifespan in C. elegans, including LIPL-4. FAs can be saturated (no double bonds), monounsaturated (MUFA, one carbon-carbon double bond) or polyunsaturated (PUFA, two or more carbon-carbon double bonds). Increasing desaturation can make FAs more susceptible to oxidation by free radicals. Elevation of Δ9 desaturases in C. elegans (FAT-5/6/7), which produce MUFAs, is associated with longevity under several conditions. Conversely, Δ5 desaturase, which can produce highly polyunsaturated FA chains, is reduced in long-lived worms. FA desaturases produce many other MUFAs and PUFAs that are present in biological samples (some examples shown in gray). Exploring how these lipid molecules determine longevity would be of great interest. FAs and other lipids can also act as signaling molecules to influence lifespan. Oleoylethanolamide (OEA), which is elevated in response to increased LIPL-4 expression in worms, activates key metabolic transcriptional regulators to extend lifespan. Lipid binding proteins mediate OEA signaling, and may be important for other lifespan extending lipid signals within cells or across tissues. ω-6 PUFAs, also elevated by increased LIPL-4 expression, can induce autophagy through unknown mechanisms.
Although these findings imply that reducing PUFAs delays aging, increasing specific PUFAs may also promote lifespan extension. In C. elegans and cell lines, supplementation with ω-6 PUFAs activates autophagy, a pro-longevity process that promotes survival under nutrient deprivation [7,8]. In worms, increased expression of lipases, which can liberate FAs from complex lipid molecules, promotes high levels of ω-6 PUFAs [8], but also of many other FAs [9]. Both induction of lipases, in particular LIPL-4 (homologous to mammalian LIPA), and increased autophagy are required for longevity in worms lacking a germline [3]. Lipases also support autophagy induction during starvation [8], raising the possibility that lipases and the free FAs they generate are important for longevity in dietary restriction. Additional genetic experiments in nematodes and mammalian cellular and organismal models, combined with lipidomics to identify relevant products of lipid metabolic enzymes such as lipases and desaturases, might shed light on the relative roles of MUFAs and PUFAs in longevity in different contexts. In addition, more work is needed to determine the effects of MUFA or PUFA supplementation on healthspan and lifespan. As supplementation experiments have yielded different results so far [6,8], it will be important to compare the effects of dose and timing of supplementation with various FAs on longevity.
Lipid signaling in lifespan regulation
Lipid signaling can systemically influence cellular functions connected to aging. Steroids are classic lipid signaling molecules that can influence fat metabolism, reproduction, and lifespan [3]. Other lipid signaling pathways may be altered to promote or curtail longevity. For example, certain sphingomyelins are enriched in the plasma of long-lived individuals and their offspring [2]. Sphingomyelins are important components of plasma membranes in the nervous system, but are also precursors for ceramides. These signaling lipids can interfere with insulin signaling [10] and have been implicated in neurodegenerative diseases [2], two disorders characteristic of aging. The accumulation of sphingomyelins may indicate reduced pro-aging ceramide signaling. The potential signaling roles of other individual lipids altered in long-lived humans [2] have yet to be explored.
Studies in C. elegans have revealed additional lipid signaling pathways that modulate lifespan. One example involves dafachronic acid, a steroid that promotes longevity in worms lacking a germline [3]. Recently, metabolomic analysis of worms overexpressing LIPL-4/LIPA identified high levels of the lipid oleoylethanolamide (OEA), which can interact with and promote transcriptional activities of the worm functional orthologs of hepatocyte nuclear factor 4 (HNF4) and peroxisome proliferator-activated receptor alpha (PPARα) (Figure 1) [9]. Interestingly, feeding worms OEA is sufficient to activate these transcriptional regulators and extend lifespan. In mammals, HNF4 and PPARs are key regulators of lipid metabolism, inflammation, and cell death [1,10]. In mice, OEA is induced by feeding, regulates satiety, and activates PPARα [1,9]. A large number of lipids have been shown to directly modulate PPAR activity [1], suggesting that PPARs and other nuclear receptors (NRs) may be key targets in pro-longevity lipid signaling pathways. Further studies into the effects of PPAR-modulating lipids on lifespan in mammals should uncover new roles for signaling lipids and NRs in aging.
How do signaling lipids reach the appropriate organelle or tissue to influence lifespan? In the case of OEA, a lipid binding protein, LBP-8, may transport this lipid to the nucleus, facilitating activation of NRs [9] (Figure 1). Recently, chemically modified lipid probes were used to identify proteins that bind common FAs [11]. This method detected many lipid carriers specific to different FAs, including FABP5/e-FABP, a fatty acid binding protein expressed in epidermal cells that interacts with PPAR-δ [12]. If candidate signaling lipids could be modified to form lipid probes, this technology could prove instrumental in understanding how lipid transport and signaling impact lifespan.
Resolving the complex relationship between fat and aging
Emerging lipidomic technologies provide exciting opportunities to understand how lipids influence lifespan. First, lipid profiling could reveal how genetic variants and metabolism are integrated in longevity. Several lipid metabolism genes have longevity-associated variants in humans, including ceramide synthase (CerS) and lipoprotein lipase (LPA) [2], which may influence lipid profiles or lipid signaling to impact longevity. Second, most of the lipid molecules associated with longevity in humans are only associated with female longevity [2]. Males and females inherently express different levels of many lipid metabolic enzymes and have distinct energetic requirements during reproductive years [2]. Intriguingly, most of the lifespan-extending mutations in C. elegans that involve altered lipid profiles or signaling also reduce reproductive fitness. Studying sex-specific differences and how lipid metabolism interacts with reproduction will be key to drawing conclusions about the roles lipids play in longevity. Third, the heritability of pro-longevity lipid profiles is of great interest. Human studies have revealed that both long-lived individuals and their children show similar lipid profiles [2], but whether this inheritance is due to genetic or epigenetic mechanisms remains unknown.
Finally, to what extent does altered lipid composition or lipid signaling support longevity under various modes of lifespan extension? Studies in C. elegans have uncovered exciting links between lipids and lifespan in many genetic and environmental interventions, which could be further explored in organisms that better model human fat metabolism and storage. In particular, mice lacking FABPs [12] would be useful in testing the contribution of lipid signaling to longevity. If general pro-longevity lipid molecules or trends were identified, would it be possible to extend lifespan by modulating the diets of wild-type organisms, including humans?
Although fat is historically associated with poor health and obesity burdens healthcare worldwide, specific lipid profiles and signals may delay aging. The continued use of lipidomics and genetic studies of lipid synthesis and signaling pathways in model organisms will undoubtedly reveal many new roles for lipids in the regulation of longevity and deepen our understanding of the complex relationship between fat and aging.
Acknowledgements
We apologize to authors of work we could not cite due to space limitations. We thank Malene Hansen, Shuo Han, and Salah Mahmoudi for helpful feedback. Supported by grants DP1AG044848 (A.B.) and T32AG0047126 (E.A.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liu S, et al. Lipid metabolites as metabolic messengers in inter-organ communication. Trends Endocrinol. Metab. 2014;25:356–363. doi: 10.1016/j.tem.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez-Covarrubias V. Lipidomics in longevity and healthy aging. Biogerontology. 2013;14:663–672. doi: 10.1007/s10522-013-9450-7. [DOI] [PubMed] [Google Scholar]
- 3.Hansen M, et al. Reproduction, Fat Metabolism, and Life Span: What Is the Connection? Cell Metab. 2013;17:10–19. doi: 10.1016/j.cmet.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mullaney BC, Ashrafi K. C. elegans fat storage and metabolic regulation. Biochim. Biophys. Acta. 2009;1791:474–478. doi: 10.1016/j.bbalip.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watts JL. Fat synthesis and adiposity regulation in Caenorhabditis elegans. Trends Endocrinol Metab. 2009;20:58–65. doi: 10.1016/j.tem.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shmookler Reis RJ, et al. Modulation of lipid biosynthesis contributes to stress resistance and longevity of C. elegans mutants. Aging. 2011;3:125–147. doi: 10.18632/aging.100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niso-Santano M, et al. Unsaturated fatty acids induce non-canonical autophagy. EMBO J. 2015;34:1025–1041. doi: 10.15252/embj.201489363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Rourke EJ, et al. ω-6 Polyunsaturated fatty acids extend life span through the activation of autophagy. Genes Dev. 2013;27:429–440. doi: 10.1101/gad.205294.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folick A, et al. Lysosomal signaling molecules regulate longevity in Caenorhabditis elegans. Science. 2015;347:83–86. doi: 10.1126/science.1258857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zechner R, et al. FAT SIGNALS - Lipases and Lipolysis in Lipid Metabolism and Signaling. Cell Metab. 2012;15:279–291. doi: 10.1016/j.cmet.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niphakis MJ, et al. A Global Map of Lipid-Binding Proteins and Their Ligandability in Cells. Cell. 2015;161:1668–1680. doi: 10.1016/j.cell.2015.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008;7:489. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brügger B. Lipidomics: Analysis of the Lipid Composition of Cells and Subcellular Organelles by Electrospray Ionization Mass Spectrometry. Annu. Rev. Biochem. 2014;83:79–98. doi: 10.1146/annurev-biochem-060713-035324. [DOI] [PubMed] [Google Scholar]