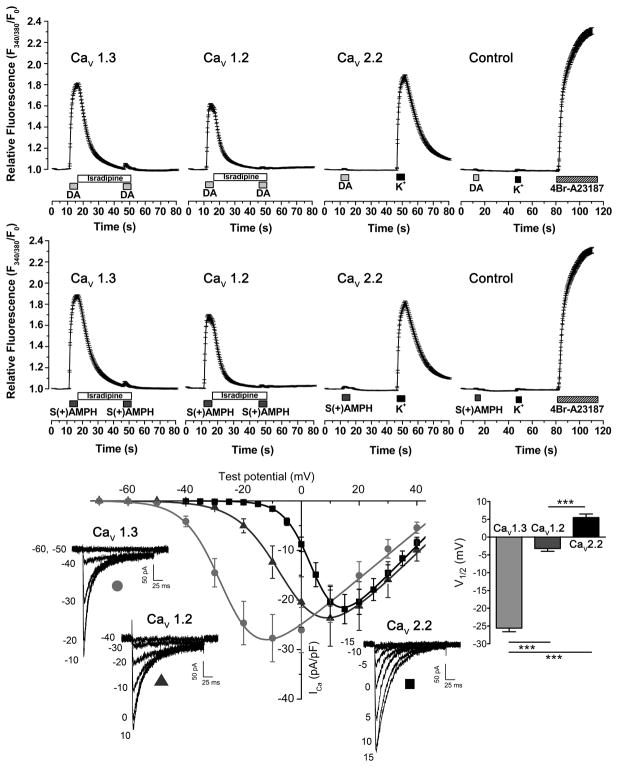
Figure 2. S(+)AMPH or DA activates CaV1.2 and CaV1.3, but not CaV2.2.
(Upper and middle panel) Intracellular Ca2+ determinations in Fura-2AM loaded Flp-hDAT cells evaluated by fluorescence microcopy, under constant perfusion and at 35°C. Flp-hDAT cells were co-transfected with CaV1.3, CaV1.2 or CaV2.2 plus β3, α2δ and EGFP plasmids. The α1 subunit was omitted from the plasmid transfection mix for the control condition. Transfected cells were identified by their EGFP signal and then briefly exposed to dopamine 10 μM (DA), S(+)AMPH 5 μM, high potassium external solution 130 mM (K+, equimolar substitution of Na+) or 4Br-A23187 (calcium ionophore, 5 μM) as indicated in the timeline of each panel. Isradipine (2 μM) averts Ca2+ signals induced by both hDAT substrates. Each trace constitutes the mean ± s.e.m. of n ≥ 81 cells per condition. (Lower panel) Voltage dependence of CaV1.2, CaV1.3 and CaV2.2- mediated Ca2+ currents: HEK293T cells were co-transfected with β3, α2δ, and EGFP expression plasmids plus alternatively CaV1.3, CaV1.2 or CaV2.2 plasmids. The Ca2+ current (ICa) recordings were carried out at room temperature under constant perfusion. Test pulses in 5 mV steps for CaV2.2 or 10 mV steps for CaV1.2 and CaV1.3 were applied from a holding potential of −80 mV. Representative responses are shown for CaV1.3 (light grey circle), CaV1.2 (dark grey triangle) and CaV2.2 (black square) and the magnitude of the test potentials are indicated in mV. The peak current density for the voltage steps were fit to Eq. 2 and yielded the following parameters: Gmax = 497 ± 86, 560 ± 128 and 631 ± 77 (pS/pF); V1/2 = −25.6 ± 1.0, −3.2 ± 0.8 and 5.5 ± 1.0 mV (***p < 0.001, one-way ANOVA, indicated in the figure); k = 6.7 ± 0.2, 7.7 ± 0.2 and 4.7 ± 0.1 (mV) for CaV1.3 (n = 8), CaV1.2 (n = 7) and CaV2.2 (n = 8), respectively.