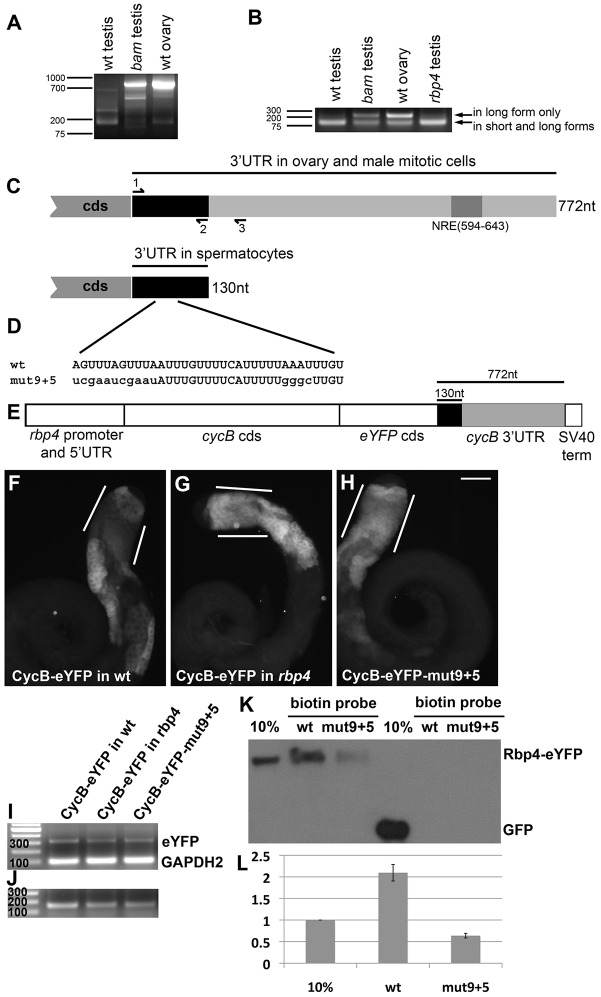
Fig. 4.
Sequences in the cycB 3′ UTR are required for translational repression. (A) 3′ RACE PCR on the cycB transcript from RNA collected from wild-type testis, wild-type ovary, bam mutant testis (spermatogonia accumulate, and spermatocytes and spermatids are absent). Forward primer for 3′ RACE was nearly identical to primer #1 in C, just 4 bases longer. (B) RT-PCR from RNA collected from wild-type testis, wild-type ovary, bam mutant testis and rbp4 mutant testis, using primers #1-3, shown in C. Predicted products: 124 bp from short and long form; 227 bp from long form only. (C) Schematic of the cycB 3′ UTR as detected in ovary and spermatogonia versus in spermatocytes. NRE, Nanos response element. (D) A conserved proportion of the short cycB 3′ UTR, with mut9+5 variation created by site-directed mutagenesis (mutated nucleotides shown in lowercase). (E) Schematic of CycB-eYFP in vivo reporter. (F-J) Anti-GFP immunofluorescence on: (F) CycB-eYFP in wild-type testis, (G) CycB-eYFP in rbp4 mutant testis and (H) CycB-eYFP-mut9+5 in wild-type testis. White lines indicate early spermatocytes. Scale bar: 100 µm in H. (I,J) RT-PCR on CycB-eYFP, CycB-eYFP in rbp4 and CycB-eYFP-mut9+5 reporters. (I) Amplifying eYFP (313-bp expected product) and (control) GAPDH2 (100 bp) to assay reporter transcript levels. (J) Using an eYFP forward primer and cycB 3′ UTR reverse primers 2 and 3. Predicted products: 157 bp from short and long form; 260 bp from long form only – not detected. (K) Anti-GFP western blot of a biotin RNA pull-down from Rbp4-eYFP or Ubi-GFP testis extract. Wild-type and mutant biotin probes as indicated. (L) Quantification via ImageJ of three independent biotin RNA pull-downs from Rbp4-eYFP. The mean of the 10% input bands was set to 1; a value of 2 for the wild-type probe indicates that that probe pulled down ∼20% of the Rbp4-eYFP input. Error bars indicate s.e.m.