Abstract
The development of organs with an epithelial parenchyma relies on reciprocal mesenchymal-epithelial communication. Mouse corneal epithelium stratification is the consequence of a coordinated developmental process based on mesenchymal-epithelial interactions. The molecular mechanism underlying these interactions remains unclear. The Wnt/β-catenin signaling pathway is involved in fundamental aspects of development through the regulation of various growth factors. Here, we show that conditional ablation of either β-catenin (Ctnnb1cKO) or co-receptors Lrp5/6 (Lrp5/6cKO) in corneal stromal cells results in precocious stratification of the corneal epithelium. By contrast, ectopic expression of a murine Ctnnb1 gain-of-function mutant (Ctnnb1cGOF) retards corneal epithelium stratification. We also discovered that Bmp4 is upregulated in the absence of β-catenin in keratocytes, which further triggers ERK1/2 (Mapk3/1) and Smad1/5 phosphorylation and enhances transcription factor p63 (Trp63) expression in mouse corneal basal epithelial cells and in a human corneal epithelial cell line (HTCE). Interestingly, mouse neonates given a subconjunctival BMP4 injection displayed a phenotype resembling that of Ctnnb1cKO. Conditional ablation of Bmp4 eradicates the phenotype produced in Ctnnb1cKO mice. Furthermore, ChIP and promoter-luciferase assays show that β-catenin binds to and suppresses Bmp4 promoter activity. These data support the concept that cross-talk between the Wnt/β-catenin/Bmp4 axis (in the stromal mesenchyme) and Bmp4/p63 signaling (in the epithelium) plays a pivotal role in epithelial stratification during corneal morphogenesis.
KEY WORDS: Precocious epithelium stratification, Mesenchymal-epithelial interactions, Ocular surface, Wnt/β-catenin signaling, Bmp4, Mouse
Summary: In mice, the canonical Wnt signaling pathway regulates Bmp4 signaling pathway activation in corneal keratocytes to modulate corneal epithelial stratification during development.
INTRODUCTION
The development of organs composed of an epithelial parenchyma is dependent on reciprocal mesenchymal-epithelial interactions. Tissue morphogenesis, cell differentiation and migration of both epithelium and mesenchyme are disrupted once the interactions are affected (Cunha et al., 2004). The vertebrate cornea is ideal for studying mesenchymal-epithelial interactions (Wilson et al., 1999). It is a transparent tissue located on the anterior ocular surface and consists of a stratified non-keratinized epithelium, a thick stroma scattered with keratocytes and a single-cell-layer endothelium (Zieske, 2004). The outermost cell layers of the corneal epithelium serve as a protective barrier against fluid loss and pathogen penetration. This biological function is established through a complex and precisely coordinated program of epithelial stratification (Koster and Roop, 2007), which is mainly dependent on communication between the epithelium and the stromal mesenchyme during development (Liu et al., 1999). It has been suggested that bidirectional mesenchymal-epithelial interactions depend on soluble growth factors/cytokines, which are tightly regulated by as yet unknown signaling transduction networks (Kao, 2010). Identification of these signaling transduction networks would facilitate our understanding of the intricate mechanism of corneal epithelium stratification at both the cellular and molecular level.
Wnt/β-catenin signaling plays a crucial role in controlling cell fate, differentiation, proliferation and apoptosis during development (Nüsslein-Volhard and Wieschaus, 1980), stem cell maintenance (Zhang et al., 2014) and adult tissue homeostasis (Logan and Nusse, 2004). This signaling activity is initiated by Wnt ligands binding to a dual receptor complex comprising Frizzled and either low-density lipoprotein receptor-related protein 5 (Lrp5) or Lrp6, resulting in the stabilization and nuclear translocation of β-catenin, which further binds with T cell factor/lymphoid enhancer factor (TCF/LEF) to regulate the transcription of downstream target genes (Clevers and Nusse, 2012). It has been reported that several ocular tissues express multiple components of the Wnt/β-catenin signaling pathway during eye development (Ang et al., 2004; Borello et al., 1999). Gain- and loss-of-function studies reveal that Wnt/β-catenin signaling is involved in coordinating vital processes during the development of ocular tissue, such as eye field formation and neural retina specification (Fuhrmann, 2008; Mukhopadhyay et al., 2006). However, the function of Wnt/β-catenin signaling in corneal development is poorly understood.
Bone morphogenetic proteins (BMPs) have been extensively studied in the regulation of cell differentiation and morphogenesis of several tissues, including the eye during development (Furuta and Hogan, 1998; Huang and Klein, 2004; Wang et al., 2014). Signaling interactions between the BMP and Wnt/β-catenin signaling pathways have been observed in fruit flies, amphibians, zebrafish and mammals (Huang and Klein, 2004; Zhu et al., 2014). For example, in Xenopus Wnt/β-catenin signaling contributes to the repression of Bmp4 on the dorsal side of the embryo and sensitizes the ectoderm to respond to neural-inducing signals (Baker et al., 1999). In the mouse dental mesenchyme, Wnt/β-catenin signaling regulates Bmp4 expression during incisor development (Fujimori et al., 2010). However, the possible cross-talk between these two signaling pathways during mouse corneal epithelium stratification has not been elucidated.
It has been shown that loss of Dkk2, an antagonist of canonical Wnt signaling pathways, suppresses corneal differentiation during mouse development (Gage et al., 2008; Mukhopadhyay et al., 2006). This strongly suggests that canonical Wnt signaling might play a crucial role in ocular surface epithelia development. In this study, we discovered that an intimate interrelationship between canonical Wnt and Bmp4 in stromal keratocytes is key to controlling the adjacent epithelial stratification during corneal development. We showed that conditional ablation of Wnt/β-catenin signaling in stromal keratocytes results in the activation of Bmp4 signaling, which led to precocious corneal epithelium stratification via upregulation of the transcription factor p63 in corneal basal epithelial cells.
RESULTS
Wnt/β-catenin signaling activity in stromal keratocytes is inversely correlated with corneal epithelium stratification
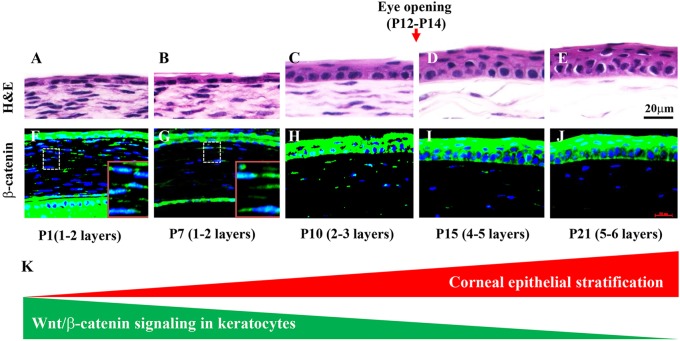
To establish baseline information regarding the activity of Wnt/β-catenin signaling in the mouse ocular surface, we took advantage of the knock-in Axin2lacZ mouse line as a reporter of canonical Wnt signaling by X-gal staining. Interestingly, we found that as the corneal epithelium stratified and more cell layers formed the activity of Wnt/β-catenin signaling gradually reduced from P1 to P21 (Fig. 1). Paraffin sections clearly revealed positive X-gal staining in the corneal epithelium, stroma and endothelium at P1. It was dramatically reduced in stromal keratocytes at P7 and disappeared at P21 (supplementary material Fig. S1). This finding was confirmed by immunofluorescence staining using a rabbit antibody (mAb D13A1) that only recognizes β-catenin that has not been phosphorylated by glycogen synthase kinase 3β (Gsk3β) and thus is functionally active in cell-cell adhesion and/or the canonical Wnt signaling pathway (Fig. 1). The β-catenin expression pattern did not change in the corneal epithelium from P1 to P21. However, nuclear localization of β-catenin in the stroma was easily detected at P1 and P7 and then steadily decreased by P21 (Fig. 1F-J).
Fig. 1.
Wnt/β-catenin activity in stromal keratocytes is inversely correlated to corneal epithelium stratification. (A-E) Corneal epithelium stratification is a postnatal event. From P1 to P7 the mouse epithelium consists of 1-2 cell layers. Just before eyelid opening at P10, the epithelium begins to stratify. At P15 it increases to 4-5 cell layers and, by P21, there are 5-6 layers. (F-J) Expression of the active form of β-catenin (immunofluorescence, green) is found in the corneal epithelium and stroma from P1 to P21. Note that the β-catenin expression pattern did not change in the corneal epithelium from P1 to P21, but exhibited a dynamic change in the stroma. Nuclear localization of β-catenin was detected in the stroma at P1 to P7 and gradually decreased through P21. Boxed regions (F,G) are shown at higher magnification in insets. Blue, cell nuclei (DAPI). (K) Schematic representation of the reduction of Wnt/β-catenin activity coinciding with epithelial stratification. Scale bars: 20 μm.
Deletion of β-catenin in stromal keratocytes promotes corneal epithelium stratification
This dynamic change of Wnt/β-catenin signaling activity in the cornea, especially in the stromal cells, prompted us to investigate its function during corneal development because there is a striking inverse correlation between Wnt/β-catenin signaling activity and the process of corneal epithelium stratification during mouse ocular surface morphogenesis. To study the potential role of Wnt/β-catenin signaling in corneal stromal keratocytes during corneal development, we employed a loss-of-function approach by crossing KR; tetO-Cre (TC) with the Ctnnb1f/f mouse line to generate an inducible triple-transgenic mouse line, KR; TC; Ctnnb1f/f, in which the Ctnnb1 gene is deleted in corneal stromal keratocytes upon Dox induction (Fig. 2A).
Fig. 2.
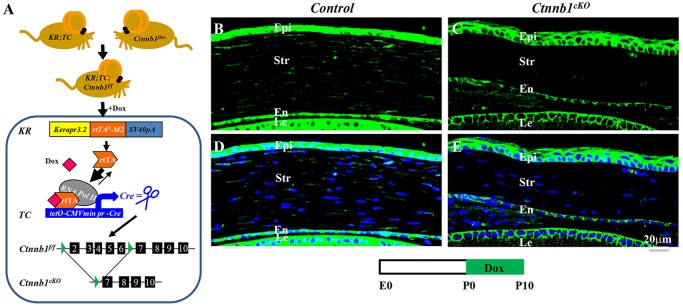
Conditional knockout of β-catenin (Ctnnb1cKO) in corneal stromal keratocytes. (A) Ablation of Ctnnb1 in keratocytes of the triple-transgenic mouse line KR; TC; Ctnnb1f/f, which was generated by crossing the Dox-inducible driver mouse KR; TC with the Ctnnb1flox mouse. Administration of Dox resulted in ablation of Ctnnb1 only in keratocan-positive keratocytes. (B-E) Ctnnb1cKO and control littermates were treated with Dox from P0 to P10. β-catenin expression (green) in stromal keratocytes was analyzed by immunofluorescence staining of corneal sections at P10. β-catenin was absent in the stroma of Ctnnb1cKO (compare C,E with B,D). DAPI, blue. epi, corneal epithelium; str, stroma; en, endothelium; le, lens.
Immunofluorescence staining confirmed that β-catenin was indeed deleted in corneal stromal keratocytes (compare Fig. 2C,E with 2B,D) upon Dox induction from P0 to P10. Surprisingly, conditional knockout of β-catenin (Ctnnb1cKO) in corneal stromal keratocytes resulted in a thicker epithelium, with 4-5 epithelial layers compared with 2-3 cell layers in littermate controls (compare Fig. 2C,E with 2B,D and supplementary material Fig. S2B with S2A). Knocking out Ctnnb1 from E12.5, prior to the expression of endogenous keratocan at E13.5 (Shiraishi et al., 1998), resulted in a more dramatic phenotype. Unlike the controls, which had 1-2 cell layers (Fig. 3A,A′), the mutant corneal epithelium had 4-5 cell layers at P0 (Fig. 3B,B′). Transmission electron microscopy (TEM) further confirmed these observations. At birth, controls had 1-2 epithelial cell layers without a clear basement membrane (Fig. 3C,C′), whereas multiple epithelial cell layers were present in the Ctnnb1cKO mutant and these showed distinct morphological differences in the various layers, i.e. basal columnar cells, suprabasal (wing) cells and flattened superficial cells (Fig. 3D). Additionally, the mutant cornea had a distinct basement membrane with the appearance of hemi-desmosomes (arrowheads in Fig. 3D′), which were not clearly defined in the control.
Fig. 3.
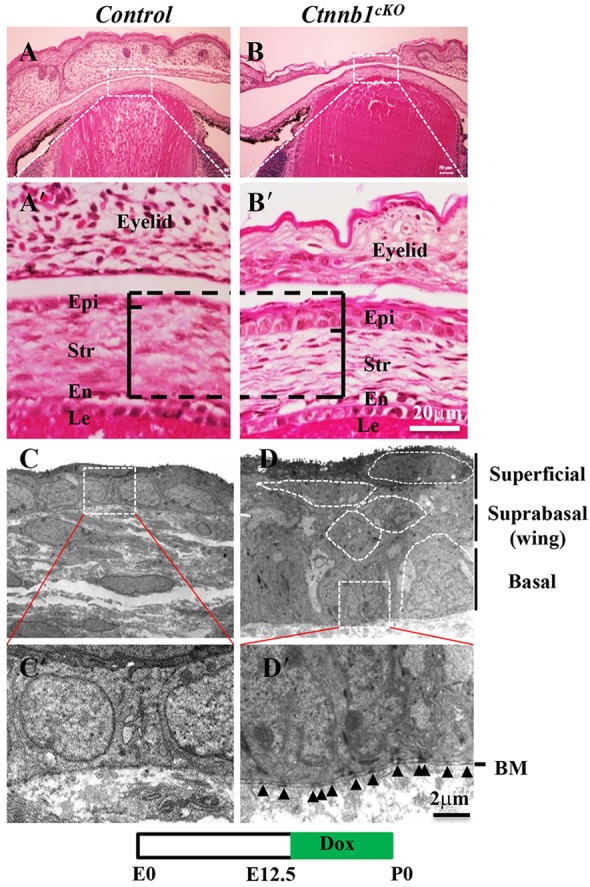
Ctnnb1 ablation in keratocytes results in precocious epithelial stratification at birth. (A-B′) H&E staining revealed that, unlike the littermate controls which had 1-2 cell layers (A,A′), the mutant corneal epithelium had 4-5 cell layers (B,B′) at birth following induction at E12.5. (C-D′) TEM images showing a 1- to 2-cell-layer corneal epithelium without a clearly defined basement membrane in controls (C,C′). By contrast, the Ctnnb1cKO mutant had multiple cell layers displaying distinct cell morphologies, i.e. basal columnar cells, suprabasal wing cells and flattened superficial cells (D). In addition, the Ctnnb1cKO mutant had a clearly defined basement membrane containing hemi-desmosomes (arrowheads in D′), which were not apparent in the control.
The expression of the corneal epithelial differentiation marker keratin 12 (Krt12) was unchanged in the Ctnnb1cKO cornea (supplementary material Fig. S2C,D). Interestingly, a triple-transgenic β-catenin gain-of-function mouse strain, KR; TC; Ctnnb1ΔE3 (Ctnnb1cGOF), that expressed a stabilized β-catenin in corneal stroma when Dox was administered from E12.5 to P21, showed an impairment of corneal epithelial stratification in that the corneal epithelium remained at 1-2 cell layers (supplementary material Fig. S3). Taken together, these data indicate that deletion of Ctnnb1 in corneal stromal keratocytes triggers precocious corneal epithelial cell stratification.
Deletion of Ctnnb1 in keratocytes promotes corneal epithelial cell proliferation
An increase in the number of epithelial cell layers in the Ctnnb1cKO mouse suggested that there was an increase in cell proliferation. To test this, immunofluorescence staining against endogenous proliferating cell nuclear antigen (Pcna) and G1/S-specific cyclin D1 (Ccnd1) was performed. Our data showed a 2-fold increase in the number of Pcna-positive cells in the basal epithelium of the Ctnnb1cKO mouse as compared with littermate controls (Fig. 4). Additionally, Ccnd1 expression was significantly increased in the Ctnnb1cKO mutant compared with littermate controls (supplementary material Fig. S4). These results indicated that loss of β-catenin in the corneal stroma results in the promotion of cell proliferation in basal epithelial cells, which is associated with precocious corneal epithelium stratification in the Ctnnb1cKO mutant.
Fig. 4.
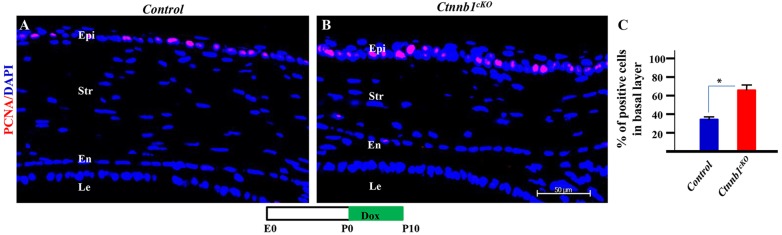
Ctnnb1 ablation in keratocytes enhances corneal epithelial cell proliferation. (A,B) Immunofluorescence micrographs of a P10 cornea probed with anti-Pcna showing that loss of Ctnnb1 in stromal keratocytes increased epithelial cell proliferation. (C) Quantification of the number of Pcna-positive cells showed a significant increase in the Ctnnb1cKO mutant. *P<0.05. Mean±s.e.m.
Bmp4 expression is upregulated in the Ctnnb1cKO mutant, which triggers corneal epithelial stratification
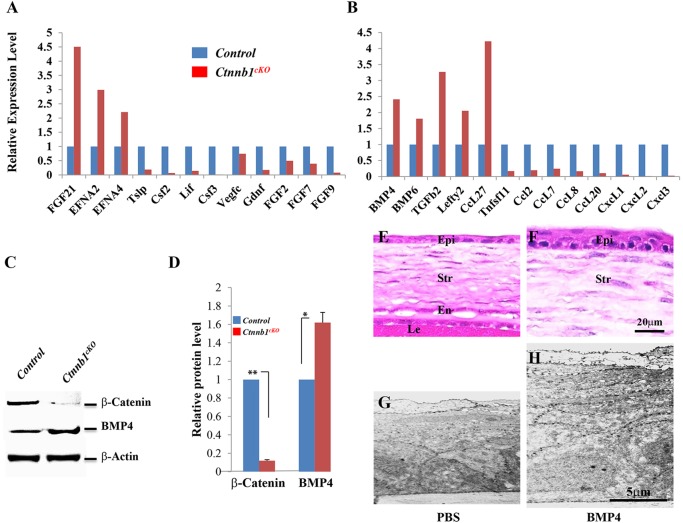
To understand the molecular mechanism(s) by which β-catenin inactivation in stromal keratocytes results in precocious corneal epithelial stratification, we screened a mouse cytokine primer library to evaluate the expression of growth factors/cytokines in primary cultured Ctnnb1cKO cells versus controls. The Ctnnb1cKO mutant cells showed changes in the expression of various cytokines and growth factors, including a 2.5-fold increase in Bmp4 expression (Fig. 5A). It has been documented that Bmp4 is a downstream target of Wnt/β-catenin signaling (Baker et al., 1999; Kim et al., 2002). The elevation of Bmp4 expression after Ctnnb1 ablation was further confirmed at the mRNA level by qRT-PCR (supplementary material Fig. S5A) and at the protein level by western blotting (Fig. 5C,D). Immunofluorescence staining showed that Bmp4 expression was upregulated in the stroma and epithelium of the Ctnnb1cKO mutants (supplementary material Fig. S5B). These data imply that Bmp4 might be the key factor that induces corneal epithelial stratification upon β-catenin inactivation in stromal keratocytes.
Fig. 5.
Differential expression of growth factors/cytokines upon Ctnnb1 ablation. (A,B) Ad-Cre-GFP-treated or Ad-GFP-treated primary cell cultures of stromal keratocytes isolated from Ctnnb1f/f mice at P0 were screened using a mouse cytokine primer library. The Ctnnb1cKO mutant showed changes in the expression of various cytokines and growth factors, including a 2.5-fold increase in Bmp4. (C) Western blotting analyses confirmed the increase in Bmp4 after Ctnnb1 ablation. β-actin provided a loading control. (D) Statistical analysis of western blot results showed that Bmp4 expression was increased at the protein level. (E-H) Histological analysis of corneas from P10 mice treated with recombinant BMP4 (10 ng/ml) or PBS (control) every other day from P0 to P8. H&E staining showed that Bmp4 administration results in an increase in the number of epithelial cells (3-4 cell layers; compare E with F). Likewise, TEM showed that BMP4 administration resulted in a much thicker epithelium (H) than that of the control (G). *P<0.05, **P<0.01. Mean±s.e.m.
To determine if exogenous Bmp4 could prompt corneal epithelial stratification in vivo, human recombinant BMP4 was injected into the subconjunctival space in C57BL/6J neonates every other day from P0 to P8 and corneal histology was examined at P10. As shown in Fig. 5, H&E staining as well as TEM images showed that addition of BMP4 resulted in an increase in the number of corneal epithelial cell layers compared with controls injected with PBS (Fig. 5E-H).
Next, we generated compound quadruple-transgenic mice, KR; TC; Ctnnb1f/f; Bmp4f/f (Bmp4cKO; Ctnnb1cKO), to examine whether simultaneous deletion of both Bmp4 and Ctnnb1 in corneal stromal keratocytes could counteract the precocious epithelial stratification seen in Ctnnb1cKO. As expected, the Bmp4cKO; Ctnnb1cKO mouse showed normal corneal epithelium stratification (supplementary material Fig. S6). These in vivo and in vitro data indicate that Bmp4 is an important mediator of the Wnt/β-catenin signaling pathway in the corneal stroma that is required for corneal epithelial cell stratification during corneal development. This also suggests that the signals that initiate corneal epithelial stratification might come from the corneal stroma.
Bmp4, as the direct effecter of Wnt/β-catenin signaling in the corneal stroma, triggers corneal epithelial stratification
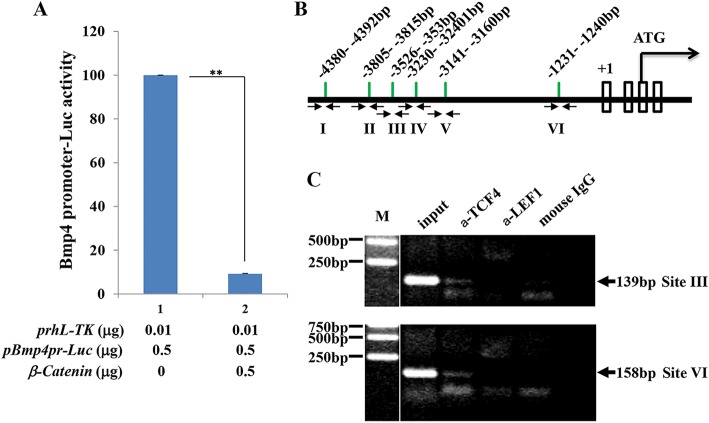
To elucidate the molecular mechanism by which β-catenin signaling regulates Bmp4 expression in corneal stromal keratocytes, we performed a luciferase assay following transient co-transfection of the mouse Bmp4 promoter (7.0 kb) and luciferase (pGL3.-Bmp4pr-Luc) plasmids into the HTK cell line. Our data showed inhibition of Bmp4 promoter activity in the presence of β-catenin. Bmp4 promoter activity was reduced to less than 10% when co-transfected with β-catenin and pGL3.-Bmp4pr-Luc (Fig. 6A). Next, NIH3T3 cells transfected with β-catenin and human TCF4 expression plasmids were used for ChIP analysis. Our data showed that the anti-TCF4 antibody was able to pull down the DNA sequence corresponding to the TCF/LEF binding site of the mouse Bmp4 promoter (Fig. 6B,C), suggesting that the complex formed by β-catenin/TCF/LEF binds to the mouse Bmp4 promoter in vivo. Together, these data indicate that Bmp4 expression is directly regulated by Wnt/β-catenin signaling.
Fig. 6.
β-catenin binds and suppresses Bmp4 promoter activity in vitro. (A) β-catenin inhibits Bmp4 promoter activity. A luciferase assay was performed by co-transfecting HTK cells with the plasmids indicated. The presence of β-catenin resulted in a 90% decrease in Bmp4 promoter activity. (B) Potential TCF/LEF binding sites (I to VI) in relation to the transcription start site (+1) are indicated within the 5 kb 5′ flanking regulatory region of mouse Bmp4. Arrows show the location of PCR primers used in the ChIP assay. (C) ChIP analysis of TCF/LEF binding to the mouse Bmp4 5′ flanking region. Note that TCF4 binds to two of the six potential sites (III and VI), while neither LEF1 nor mouse IgG bound to any of the sites in the Bmp4 5′ flanking regulatory region. M, DNA size marker. **P<0.01. Mean±s.e.m.
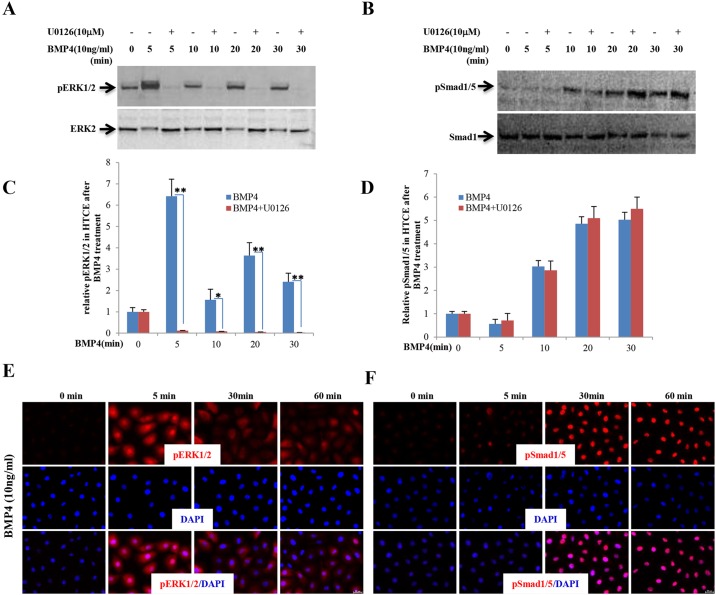
Bmp4, as a member of the TGFβ superfamily, binds to Bmpr1 and Bmpr2, transmitting signals via Smad and/or the MAP kinase pathways to effect target genes (Miyazono et al., 2010). Next, we attempted to determine which signaling pathway Bmp4 signals through to regulate corneal epithelial stratification. HTCE cells were treated with human recombinant BMP4 followed by analysis by western blot and immunofluorescence staining. Western blot data clearly showed that the phosphorylation levels of both ERK1/2 (Mapk3/1 – Mouse Genome Informatics) and Smad1/5 were transiently increased at different time points after BMP4 protein treatment (Fig. 7A,B). ERK1/2 responded to BMP4 more rapidly than Smad1/5 (Fig. 7A-D), which is consistent with the results from immunofluorescence staining (Fig. 7E,F). Collectively, the in vivo and in vitro data indicated that, in the absence of β-catenin in the corneal stromal keratocytes, Bmp4 suppression is lifted allowing it to serve as a paracrine factor triggering corneal epithelial stratification.
Fig. 7.
BMP4 triggers ERK1/2 and Smad1/5 phosphorylation in HTCE cells. (A,B) Dynamic changes in phosphorylation of ERK1/2 and Smad1/5 in HTCE cells after BMP4 treatment were confirmed by western blot. ERK1/2 responded to BMP4 treatment within 5 min and then became dephosphorylated, whereas Smad1/5 phosphorylation began after 10 min treatment. The specific MEK inhibitor U0126 impeded ERK1/2 phosphorylation but had no effect on Smad1/5 phosphorylation. (C,D) Statistical analysis showing the ratios of phosphorylated versus non-phosphorylated ERK1/2 and Smad1/5. (E,F) Immunocytofluorescence staining confirmed the dynamic change in phosphorylation of ERK1/2 and Smad1/5 in HTCE cells triggered by BMP4. *P<0.05, **P<0.01. Mean±s.e.m.
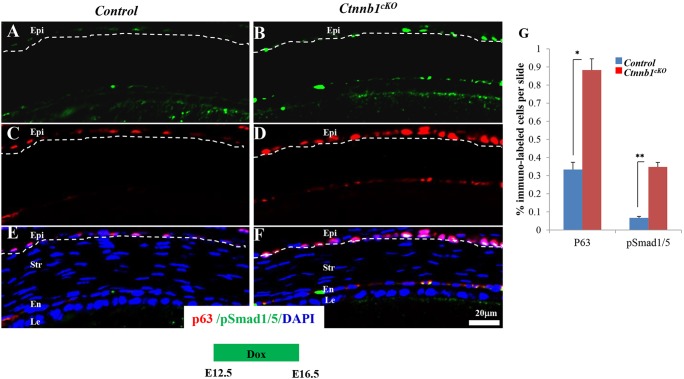
The activation of Bmp4 signaling in the corneal stroma caused by deletion of β-catenin enhances p63 expression in corneal epithelial basal cells
It has been established that the transcription factor p63 (Trp63 – Mouse Genome Informatics) is essential for initiating epithelial stratification during cornea and skin development and for maintaining the proliferative potential of the basal layer keratinocytes (Koster et al., 2004; Ng et al., 2013; Zhu et al., 2014). Two main isoforms are encoded by the p63 gene: TAp63 and ΔNp63 (Yang et al., 1998). To explore the potential involvement of p63 in the precocious corneal epithelial stratification observed in Ctnnb1cKO mutants, immunofluorescence staining with antibody against the two isoforms of p63 was performed to detect p63 expression, induced from E0 to E16.5. The data showed a concomitant increase in Smad1/5 and ERK1/2 phosphorylation, as well as an increase in p63-positive cells, in the mutant corneal epithelium when compared with littermate controls (Fig. 8G, also compare Fig. 8A,C,E with 8B,D,F and supplementary material Fig. S7A,C,E with S7B,D,F). Likewise, p63 expression was upregulated in mouse corneal basal epithelial cells subcutaneously injected with BMP4 protein (supplementary material Fig. S8A,B). Furthermore, ΔNp63 expression was significantly elevated in HTCE cells treated with different dosages of BMP4 protein (supplementary material Fig. S8C,D). These data suggested that lifting the suppression of Bmp4 signaling in Ctnnb1cKO mice resulted in upregulation of p63 expression, which promoted corneal epithelial stratification.
Fig. 8.
Ctnnb1 ablation in keratocytes results in Smad1/5 phosphorylation and enhances p63 expression. (A-F) Immunofluorescence staining showed that at E16.5 more p63-labeled basal cells and more pSmad1/5-labeled basal cells were detected in Ctnnb1cKO mutant corneal epithelium than in the littermate controls. Note that p63 expression was increased in the mutant and that this colocalized with Smad1/5 phosphorylation. The dashed line delineates the corneal epithelium layer. (G) Statistical analysis of the immunofluorescence staining showed that Smad1/5 phosphorylation and p63 expression were upregulated in the mutant. *P<0.05, **P<0.01. Mean±s.e.m.
Wnt/β-catenin signaling in keratocytes negatively regulates Bmp4 signaling in the epithelium during corneal development
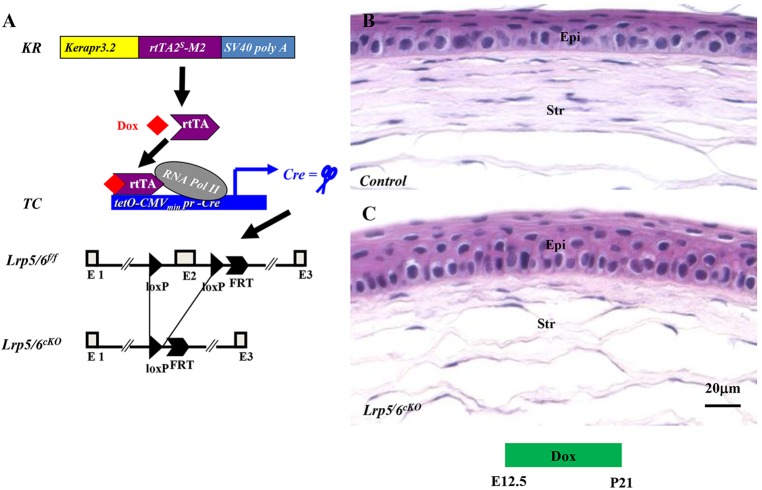
It is well documented that β-catenin is the core mediator of the canonical Wnt signaling pathway, while Lrp5 and Lrp6 are the co-receptors of Wnt ligands. Therefore, knockout of either β-catenin or Lrp5/Lrp6 will block the Wnt signaling pathway. We asked whether the phenotype observed in the Ctnnb1cKO line could be recapitulated in mice lacking Lrp5/Lrp6 in the stromal keratocytes. KR; TC; Lrp5f/f; Lrp6f/f transgenic mice were generated by natural mating between KR, TC, Lrp5f/f and Lrp6f/f mice (Fig. 9A). No overt phenotype was observed in the Lrp5 or Lrp6 single-knockout mice (data not shown); however, loss of both Lrp5 and Lrp6 resulted in a phenotype similar to that observed in the Ctnnb1cKO mutant (compare Fig. 9C with 9B). These data suggest that the canonical Wnt/β-catenin signaling pathway in cornea stromal keratocytes plays a pivotal role in triggering corneal epithelial stratification.
Fig. 9.
Deletion of Lrp5 and Lrp6 in stromal keratocytes recapitulates the Ctnnb1cKO phenotype. (A) Schematic representation of Dox-dependent deletion of Lrp5 and Lrp6 in stromal keratocytes via the Cre-loxP system. (B,C) A thicker epithelium is observed in Lrp5cKO and Lrp6cKO (Lrp5/6cKO) double mutants (C) than in littermate controls (B).
DISCUSSION
Our results provide, for the first time, direct genetic evidence that Wnt/β-catenin signaling in stromal keratocytes regulates corneal epithelial stratification via the Bmp4→p63 axis. Using compound transgenic mouse models we demonstrated that inactivation of the canonical Wnt signaling pathway in cornea stromal keratocytes via conditional disruption of the key mediator β-catenin or of co-receptors Lrp5/6 of the Wnt signaling pathway can lift the repression of Bmp4 in a naive situation. Following derepression, Bmp4 then acts as a paracrine growth factor triggering corneal epithelial stratification through upregulation of transcription factor p63 expression. These data present an excellent example of the coordinated interaction of the stroma and epithelium by means of cross-talk between the Wnt/β-catenin and Bmp4 signaling pathways, which is essential for corneal epithelial maturation.
Multiple components of the canonical Wnt signaling pathway are expressed in the developing ocular surface (Ang et al., 2004; Borello et al., 1999; Liu et al., 2003); however, in the cornea the exact components of this pathway are not well characterized. In this study, we found that canonical Wnt signaling is dynamically decreased in the cornea stroma from P1 to P21, as revealed by whole-mount X-gal staining of the Axin2lacZ mouse cornea and immunofluorescence staining for the active form of β-catenin. This dynamic change in activity is in agreement with the notion that canonical Wnt signaling must be repressed in the cornea for proper development, as reported for the Dkk2 knockout mouse (Mukhopadhyay et al., 2006) and in Pitx2-deficient mice (Gage et al., 2008). In addition, it has been shown that ectopic expression of the stable form of β-catenin in the differentiated cornea epithelial cells using Krt12 rtTA driver mice leads to corneal intraepithelial neoplasia (Zhang et al., 2010). In the current study, loss of β-catenin in the cornea stroma resulted in precocious epithelial stratification. Furthermore, canonical Wnt/β-catenin signaling activity forms a gradient from the peripheral limbal region to the central cornea (supplementary material Fig. S1). This suggests that canonical Wnt/β-catenin signaling has to be precisely and stringently controlled in the stroma for corneal epithelial stratification.
Our current focus considers the canonical Wnt signaling pathway and its regulation in the corneal stroma during development. A variety of secreted Wnt inhibitors, including Dkk2, have been identified that regulate Wnt signaling either by binding to Wnt ligands or to their receptors (Hsieh et al., 1999; Mao and Niehrs, 2003; Rattner et al., 1997). It has previously been reported that Dkk2 expression in the corneal and limbal epithelium is required to control Wnt activity in the stroma, which contributes to the formation of the corneal epithelium (Mukhopadhyay et al., 2006). Based on all these observations, we propose that Dkk2 in the corneal epithelium may modulate the activity of Wnt/β-catenin signaling in the stroma, which is essential for releasing the inhibition of Bmp4 expression. Bmp4, in turn, prompts corneal epithelial stratification through p63. Therefore, the signaling communication involved in corneal epithelial stratification via the Wnt signaling pathway may follow ‘epithelium-stroma-epithelium’ interaction. This will be explored in the future via in vitro and in vivo experiments that include utilizing compound transgenic mouse models to modify the expression of Wnt signaling inhibitors, such as Dkk2, in the corneal epithelium.
The Wnt signaling pathway is essential for the regulation of multiple processes during eye development, such as tissue specification, morphogenetic movement, cellular proliferation and differentiation (Fuhrmann, 2008). Most of the previous genetic studies examining the role of Wnt/β-catenin signaling in the cornea have been carried out in the mouse corneal epithelium (Chikama et al., 2008; Mukhopadhyay et al., 2006; Zacharias and Gage, 2010; Zhang et al., 2010). We took advantage of a unique transgenic mouse driver line, KR [in which a keratocan gene regulatory cassette drives a mutant reverse tetracycline transactivator minigene (rtTA)], to delete β-catenin and/or Lrp5/6 specifically in corneal stromal keratocytes and, surprisingly, observed a phenotype: mutant neonates exhibited a thicker corneal epithelium. We also noticed an anomaly in the mutant eyelid (Fig. 3B) that caused eyelid opening at P1 to P5 (data not shown). Earlier eyelid opening in the neonatal mutant is unlikely to play a role in the formation of the thicker corneal epithelium because we observed a similar corneal epithelium phenotype in mutant mice when induced after eyelid fusion (Fig. 2; supplementary material Fig. S2).
In our cytokine cDNA library screening experiment, in addition to the upregulation of Bmp4, we observed simultaneous trends in the upregulation of cytokines and growth factors, such as fibroblast growth factor 21 (Fgf21), chemokine (c-c motif) ligand 27 (Ccl27), ephrin A2 (Efna2) and ephrin A4 (Efna4). Fgf21 acts as a metabolic regulator and plays a key role as an anti-diabetic and anti-obesity agent (Kharitonenkov et al., 2005; Woo et al., 2013). Ccl27 plays a role in T cell-mediated inflammation in the skin (Homey et al., 2002). Based on their function, it is unlikely that Fgf21 and Ccl27 are directly involved in the precocious corneal epithelial stratification seen in the absence of β-catenin. Nonetheless, Wnt signaling is essential for neural development and corneal nerve fibers exert important trophic influences on the corneal epithelium (Ille and Sommer, 2005). Efna2 and Efna4, which are membrane-bound proteins, play a crucial role during the development of the nervous system (Flanagan and Vanderhaeghen, 1998). The upregulation of Efna2 and Efna4 in the β-catenin mutant cells might not be related to corneal epithelial stratification. Among all of the upregulated cytokines in the β-catenin mutant, only Bmp4 has previously been reported to be a downstream target of Wnt/β-catenin signaling (Baker et al., 1999; Fujimori et al., 2010). Therefore, we addressed the possible involvement of Bmp4 in the observed phenotype and found that Bmp4 indeed plays an important role in corneal epithelium development. Nevertheless, further in-depth investigation will be required to establish whether any of the additional factors mentioned above, which are impacted in transcriptional regulation by β-catenin, has any precise function in corneal development.
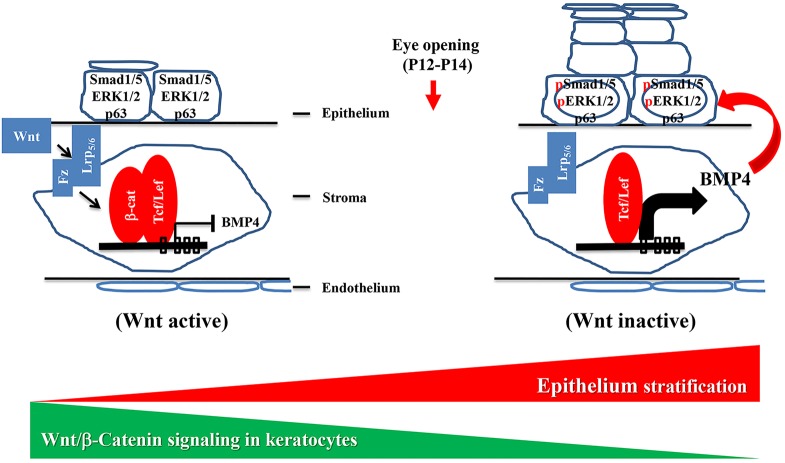
Our data from in vitro and in vivo experiments suggest that the change in Bmp4 expression in β-catenin mutant mice may be the consequence of loss of Wnt/β-catenin signaling. This implies that during corneal development communication between the stroma and the epithelium is essential for the initiation of corneal epithelial stratification. We propose a model whereby cross-talk between the Wnt/β-catenin and Bmp4 signaling pathways in the stroma and epithelium is essential for mouse corneal development (Fig. 10). Under normal developmental conditions, Wnt/β-catenin signaling is active in the cornea stromal keratocytes at early developmental stages and this is responsible for suppressing Bmp4 expression in the keratocytes. Before eyelid opening, Wnt/β-catenin signaling is gradually inactivated by some unknown mechanism, which results in lifting of the inhibition and an increase in Bmp4 expression. Bmp4, as a paracrine growth factor, then stimulates p63 expression in the corneal epithelium, which initiates corneal epithelial stratification.
Fig. 10.
Model of the interaction between corneal stroma (canonical Wnt signaling) and epithelium (Bmp4 signaling) during mouse corneal development. Before stratification (left), Wnt/β-catenin signaling is active, which suppresses Bmp4 expression. Before eyelid opening (right), the Wnt/β-catenin signaling pathway is downregulated leading to the relief of Bmp4 suppression. Bmp4 then triggers epithelial cell stratification via p63. Fz, Frizzled.
MATERIALS AND METHODS
Mouse strains and genotyping
The genetically modified mouse lines KR (keratocan promoter-driven rtTA; Zhang et al., 2011), tetO-Cre (Perl et al., 2002), Ctnnb1flox (Brault et al., 2001), Ctnnb1floxE3 (Harada et al., 1999), Bmp4flox (Liu et al., 2004), Axin2lacZ (Lustig et al., 2002), Lrp5flox (Joeng et al., 2011) and Lrp6flox (Joeng et al., 2011) have been described previously. Compound transgenic mice were generated via natural mating. All mice were bred at the Animal Facility of the University of Cincinnati Medical Center. Experimental procedures for handling mice were approved by the Institutional Animal Care and Use Committee, University of Cincinnati/College of Medicine. Transgene alleles were identified by PCR genotyping with tail DNA using specific primer pairs (supplementary material Table S1).
Administration of doxycycline and recombinant BMP4 protein
To induce the conditional knockout of Ctnnb1, Lrp5/6, Bmp4 or ectopic expression of Ctnnb1cGOF in mice from embryonic day (E) 12.5 to various developmental stages, including E16.5, postnatal day (P) 0, P10 and P21, pregnant dams were given an intraperitoneal (IP) injection of doxycycline [Dox; 80 μg/g body weight in 1× phosphate buffered saline (PBS), pH 7.4; Clontech Laboratories] followed by administration of Dox chow (Dox diet #AD3008S, Custom Animal Diets, Bangor, PA, USA) ad libitum. Neonatal mice at birth were induced through milk-nursing following a Dox IP injection of the mother and feeding with Dox chow ad libitum. Control animals were littermates with either single or double transgene(s).
To administer BMP4 protein, C57BL/6J neonates were given a subconjunctival injection with human recombinant BMP4 protein (10 ng/ml in PBS, 20 µl per eye; R&D Systems, 314-BP/CF) every 48 h from P0 to P8. Mice were sacrificed at P10 and the enucleated eyeballs were subjected to immunohistochemical examination.
X-gal staining for detection of β-galactosidase activity
Enucleated eyes were fixed in 4% paraformaldehyde in 1× PBS pH 7.4 (PFA/PBS) for 30 min at 4°C followed by incubation in X-gal staining solution (5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 2 mM MgCl2, 0.02% NP-40, 0.01% sodium deoxycholate, 0.4 mg/ml X-gal in PBS) overnight at room temperature. The eyes were postfixed in 4% PFA/PBS overnight at 4°C and examined by microscopy as whole-mount tissue or paraffin sections.
Histological analysis
Mouse samples were fixed overnight in 4% PFA/PBS, followed by paraffin embedding. Deparaffinized sections (5 µm) were stained with Hematoxylin and Eosin (H&E).
Transmission electron microscopy (TEM)
Mouse cornea samples were fixed in 0.1 M cacodylate buffer (pH 7.4) containing 3% glutaraldehyde and 2% paraformaldehyde for 2 h at 4°C and then preserved in 0.1 M cacodylate buffer (pH 7.4) containing 0.5% glutaraldehyde at 4°C overnight. After refixation in 1% osmium tetraoxide for 1 h at 4°C, cornea samples were washed in 0.1 M cacodylate buffer (pH 7.4) for 10 min three times, and then dehydrated in a graded ethanol series and embedded in Epon 812 epoxy resin (Electron Microscopy Sciences, #14120). Ultrathin 50 nm sections were stained with uranyl acetate and lead citrate and images were photographed with a Hitachi 7500 transmission electron microscope equipped with an AMT digital camera.
Mouse corneal stroma fibroblast primary cell culture
Corneal epithelium and Descemet's membrane were removed from the cornea of newborn mice Ctnnb1flox/flox (Ctnnb1f/f) with a scalpel blade. The remaining stromal tissue was digested overnight with 2.0 mg/ml collagenase and 0.5 mg/ml hyaluronidase in DMEM (Gibco-BRL) at 37°C. Isolated cells were washed in DMEM and cultured in DMEM supplemented with 10% fetal calf serum (Gibco-BRL) and 1% penicillin-streptomycin (Gibco-BRL) for 48 h, and then replaced with DMEM containing 10% fetal bovine serum without antibiotics.
Immunohistofluorescence staining
Tissue sections (5 µm) were deparaffinized, rehydrated in a graded ethanol series and subjected to antigen retrieval in sodium citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0) with boiling for 30 min. Corneal sections were then blocked with 3% bovine serum albumin in PBS containing 0.05% NP-40 for 1 h at room temperature, then incubated overnight at 4°C with primary antibodies diluted in the same buffer. After three washes in PBS containing 0.1% Tween 20 (PBST), slides were incubated at room temperature for 1 h with Alexa Fluor 488- and/or Alexa Fluor 555-conjugated secondary antibodies (Life Technologies) and 1 µg/ml DAPI as a nuclear counterstain. Following incubation, the slides were washed with PBST and mounted with Mowiol (Sanofi-Aventis). Sections were examined and photographed using a Zeiss Axio Observer Z1 microscope equipped with an Axiocam Mrm camera. For data acquisition, we used Axiovision 4.6 software (Zeiss). Antibodies used are listed in supplementary material Table S2.
Western blotting analysis
Human HTCE cells, human HTK immortalized cells (Jester et al., 2003) or corneal stroma fibroblast primary cells were lysed in lysis buffer [50 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.1% SDS, 1% NP-40, 0.5% deoxycholate, 1× protease inhibitor cocktail (Sigma P8340)]. Frozen tissue was homogenized in RIPA buffer containing phosphatase and protease inhibitors. Protein lysates (20 µg) from each sample were separated on a 4-20% linear gradient Tris-HCl denaturing polyacrylamide Ready Gel (Bio-Rad) and transferred to PVDF membrane (Whatman). Primary antibodies were incubated in 5% nonfat dried milk in TBST (10 mM Tris-HCl pH 8.0, 150 mM NaCl, 0.05% Tween 20) at 4°C overnight. After three washes in PBST, Alexa Fluor 488- and/or Alexa Fluor 555-conjugated secondary antibodies (Life Technologies) were incubated at room temperature for 1 h. Following three washes in PBST, samples were examined and photographed using a VersaDoc 4000MP imaging system (Bio-Rad). Antibodies are listed in supplementary material Table S2.
Cytokine expression screening and quantitative real-time PCR (qRT-PCR)
Mouse corneal stroma fibroblast primary cells were infected with adenovirus Ad-Cre-GFP for 4 days to delete the Ctnnb1 gene. Cells were infected with Ad-GFP as a control. Total RNA (10 µg) was isolated from virus-infected cells using TRIzol RNA isolation reagents (Life Technologies) then annealed to random primers and reverse transcribed with an avian reverse transcriptase (RT) kit (Promega) according to the manufacturer's instructions. cDNA was subjected to RT-PCR-based gene expression screening using a mouse cytokine primer library (RealTimePrimers, #MCA-II). qRT-PCR was performed using the CFX96 real-time system on a C1000 thermal cycler (Bio-Rad). After the initial step at 95°C for 3 min, 40 cycles were performed at 95°C for 15 s, 62°C for 15 s, 72°C for 20 s. The cycle threshold values were used to calculate the normalized expression of genes of interest against Gapdh using QGene software. RT-qPCR primer sets are listed in supplementary material Table S3.
Chromatin immunoprecipitation (ChIP) assay
Murine NIH3T3 cells (ATCC® CRL-1658™) were co-transfected with 20 µg pcDNA3-β-catenin plasmid (Kolligs et al., 1999) and pcDNA/Myc-TCF4 (Korinek et al., 1997) using GeneJammer transfection reagent (Invitrogen). 48 h post-transfection, cells were cross-fixed with 1% formaldehyde at 37°C for 10 min and subjected to ChIP assay with antibodies against TCF4 (Cell Signaling, 2569) using the ChIP Assay Kit (Millipore, 17-295) following the manufacturer's instructions. Purified DNA post-ChIP was used as the template in PCR to verify the interaction between DNA and protein. Primer sets are listed in supplementary material Table S3.
Promoter-luciferase assay
HTK cells seeded in six-well plates at 80% confluence were transiently transfected using GeneJammer transfection reagent with a mixture of three different plasmids: (1) prhL-TK (Promega), which served as the internal control; (2) pGL3.0-Basic vector (Promega) or recombinant plasmid harboring the mouse Bmp4 promoter pGL3.0-mBmp4pr (Yasunaga et al., 2011); (3) the cDNA expression plasmid pcDNA3-β-catenin (Kolligs et al., 1999) or pcDNA/Myc-TCF4 (Korinek et al., 1997). 48 h post-transfection, cells were collected in 1× passive lysis buffer (Promega). The luciferase assay was conducted using the Dual Luciferase Reporter (DLR) Assay System (Promega) according to the manufacturer's recommendations. Luminescence was measured using a Glomax multidetection system (Promega).
Statistical analysis
Two-tailed Student's t-test was performed in Excel (Microsoft). P<0.05 was considered statistically significant. All quantification data are presented as mean±s.e.m.
Acknowledgements
We thank Dr Bart O. Williams (Center for Skeletal Disease Research and Laboratory of Cell Signaling and Carcinogenesis, Van Andel Research Institute, 333 Bostwick NE Grand Rapids, MI 49503, USA) for kindly giving Lrp5flox and Lrp6flox mouse lines for this study; Hui-Chun Kung, Ya-Ling Chen and Shih-Hsin Hsiao for preparation of TEM studies at the Microscope Center of Chang-Gung Memorial Hospital; and Mr Richard Converse for proofreading the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Y.Z. and C.-Y.L. designed the study and wrote the manuscript. Y.Z. and S.Z. performed most of the in vivo experiments and data analysis. L.-K.Y. prepared the TEM images. Y.Y., M.C. and M.Y. performed the in vitro experiments. W.W.-Y.K. modified the manuscript.
Funding
This study was supported by grants from the National Institutes of Health/National Eye Institute (NIH/NEI) [RO1 EY21501 to C.-Y.L. and EY13755 to W.W.-Y.K.], Research to Prevent Blindness and Ohio Lions Foundation for Eye Research. This study was supported in part by National Science Council Grants (Taiwan) [1012314B182A056MY3 and 104-2314B182A097MY3 to L.-K.Y.]. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.125393/-/DC1
References
- Ang S. J., Stump R. J. W., Lovicu F. J. and McAvoy J. W. (2004). Spatial and temporal expression of Wnt and Dickkopf genes during murine lens development. Gene Expr. Patterns 4, 289-295. 10.1016/j.modgep.2003.11.002 [DOI] [PubMed] [Google Scholar]
- Baker J. C., Beddington R. S. P. and Harland R. M. (1999). Wnt signaling in Xenopus embryos inhibits bmp4 expression and activates neural development. Genes Dev. 13, 3149-3159. 10.1101/gad.13.23.3149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borello U., Buffa V., Sonnino C., Melchionna R., Vivarelli E. and Cossu G. (1999). Differential expression of the Wnt putative receptors Frizzled during mouse somitogenesis. Mech. Dev. 89, 173-177. 10.1016/S0925-4773(99)00205-1 [DOI] [PubMed] [Google Scholar]
- Brault V., Moore R., Kutsch S., Ishibashi M., Rowitch D. H., McMahon A. P., Sommer L., Boussadia O. and Kemler R. (2001). Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128, 1253-1264. [DOI] [PubMed] [Google Scholar]
- Chikama T., Liu C.-Y., Meij J. T. A., Hayashi Y., Wang I.-J., Yang L., Nishida T. and Kao W. W. Y. (2008). Excess FGF-7 in corneal epithelium causes corneal intraepithelial neoplasia in young mice and epithelium hyperplasia in adult mice. Am. J. Pathol. 172, 638-649. 10.2353/ajpath.2008.070897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. and Nusse R. (2012). Wnt/β-catenin signaling and disease. Cell 149, 1192-1205. 10.1016/j.cell.2012.05.012 [DOI] [PubMed] [Google Scholar]
- Cunha G. R., Cooke P. S. and Kurita T. (2004). Role of stromal-epithelial interactions in hormonal responses. Arch. Histol. Cytol. 67, 417-434. 10.1679/aohc.67.417 [DOI] [PubMed] [Google Scholar]
- Flanagan J. G. and Vanderhaeghen P. (1998). The ephrins and Eph receptors in neural development. Annu. Rev. Neurosci. 21, 309-345. 10.1146/annurev.neuro.21.1.309 [DOI] [PubMed] [Google Scholar]
- Fuhrmann S. (2008). Wnt signaling in eye organogenesis. Organogenesis 4, 60-67. 10.4161/org.4.2.5850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimori S., Novak H., Weissenböck M., Jussila M., Gonçalves A., Zeller R., Galloway J., Thesleff I. and Hartmann C. (2010). Wnt/β-catenin signaling in the dental mesenchyme regulates incisor development by regulating Bmp4. Dev. Biol. 348, 97-106. 10.1016/j.ydbio.2010.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y. and Hogan B. L. M. (1998). BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 12, 3764-3775. 10.1101/gad.12.23.3764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. J., Qian M., Wu D. and Rosenberg K. I. (2008). The canonical Wnt signaling antagonist DKK2 is an essential effector of PITX2 function during normal eye development. Dev. Biol. 317, 310-324. 10.1016/j.ydbio.2008.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N., Tamai Y., Ishikawa T., Sauer B., Takaku K., Oshima M. and Taketo M. M. (1999). Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 18, 5931-5942. 10.1093/emboj/18.21.5931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homey B., Alenius H., Müller A., Soto H., Bowman E. P., Yuan W., McEvoy L., Lauerma A. I., Assmann T., Bünemann E. et al. (2002). CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat. Med. 8, 157-165. 10.1038/nm0202-157 [DOI] [PubMed] [Google Scholar]
- Hsieh J.-C., Kodjabachian L., Rebbert M. L., Rattner A., Smallwood P. M., Samos C. H., Nusse R., Dawid I. B. and Nathans J. (1999). A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature 398, 431-436. 10.1038/18899 [DOI] [PubMed] [Google Scholar]
- Huang H.-C. and Klein P. S. (2004). Interactions between BMP and Wnt signaling pathways in mammalian cancers. Cancer Biol. Ther. 3, 676-678. 10.4161/cbt.3.7.1026 [DOI] [PubMed] [Google Scholar]
- Ille F. and Sommer L. (2005). Wnt signaling: multiple functions in neural development. Cell. Mol. Life Sci. 62, 1100-1108. 10.1007/s00018-005-4552-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester J. V., Huang J., Fisher S., Spiekerman J., Chang J. H., Wright W. E. and Shay J. W. (2003). Myofibroblast differentiation of normal human keratocytes and hTERT, extended-life, human corneal fibroblasts. Invest. Ophthal. Vis. Sci. 44, 1850-1858. 10.1167/iovs.02-0973 [DOI] [PubMed] [Google Scholar]
- Joeng K. S., Schumacher C. A., Zylstra-Diegel C. R., Long F. and Williams B. O. (2011). Lrp5 and Lrp6 redundantly control skeletal development in the mouse embryo. Dev. Biol. 359, 222-229. 10.1016/j.ydbio.2011.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao W. W.-Y. (2010). Corneal morphogenesis during development and diseases. Eye Contact Lens 36, 265-268. 10.1097/ICL.0b013e3181ef0e00 [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A., Shiyanova T. L., Koester A., Ford A. M., Micanovic R., Galbreath E. J., Sandusky G. E., Hammond L. J., Moyers J. S., Owens R. A. et al. (2005). FGF-21 as a novel metabolic regulator. J. Clin. Invest. 115, 1627-1635. 10.1172/JCI23606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. S., Crooks H., Dracheva T., Nishanian T. G., Singh B., Jen J. and Waldman T. (2002). Oncogenic beta-catenin is required for bone morphogenetic protein 4 expression in human cancer cells. Cancer Res. 62, 2744-2748. [PubMed] [Google Scholar]
- Kolligs F. T., Hu G., Dang C. V. and Fearon E. R. (1999). Neoplastic transformation of RK3E by mutant beta-catenin requires deregulation of Tcf/Lef transcription but not activation of c-myc expression. Mol. Cell. Biol. 19, 5696-5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek V., Barker N., Morin P. J., van Wichen D., de Weger R., Kinzler K. W., Vogelstein B. and Clevers H. (1997). Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science 275, 1784-1787. 10.1126/science.275.5307.1784 [DOI] [PubMed] [Google Scholar]
- Koster M. I. and Roop D. R. (2007). Mechanisms regulating epithelial stratification. Annu. Rev. Cell Dev. Biol. 23, 93-113. 10.1146/annurev.cellbio.23.090506.123357 [DOI] [PubMed] [Google Scholar]
- Koster M. I., Kim S., Mills A. A., DeMayo F. J. and Roop D. R. (2004). p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev. 18, 126-131. 10.1101/gad.1165104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. J., Kao W. W.-Y. and Wilson S. E. (1999). Corneal epithelium-specific mouse keratin K12 promoter. Exp. Eye Res. 68, 295-301. 10.1006/exer.1998.0593 [DOI] [PubMed] [Google Scholar]
- Liu H., Mohamed O., Dufort D. and Wallace V. A. (2003). Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev. Dyn. 227, 323-334. 10.1002/dvdy.10315 [DOI] [PubMed] [Google Scholar]
- Liu W., Selever J., Wang D., Lu M.-F., Moses K. A., Schwartz R. J. and Martin J. F. (2004). Bmp4 signaling is required for outflow-tract septation and branchial-arch artery remodeling. Proc. Natl. Acad. Sci. USA 101, 4489-4494. 10.1073/pnas.0308466101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan C. Y. and Nusse R. (2004). The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781-810. 10.1146/annurev.cellbio.20.010403.113126 [DOI] [PubMed] [Google Scholar]
- Lustig B., Jerchow B., Sachs M., Weiler S., Pietsch T., Karsten U., van de Wetering M., Clevers H., Schlag P. M., Birchmeier W. et al. (2002). Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol. Cell. Biol. 22, 1184-1193. 10.1128/MCB.22.4.1184-1193.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao B. and Niehrs C. (2003). Kremen2 modulates Dickkopf2 activity during Wnt/LRP6 signaling. Gene 302, 179-183. 10.1016/S0378-1119(02)01106-X [DOI] [PubMed] [Google Scholar]
- Miyazono K., Kamiya Y. and Morikawa M. (2010). Bone morphogenetic protein receptors and signal transduction. J. Biochem. 147, 35-51. 10.1093/jb/mvp148 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay M., Gorivodsky M., Shtrom S., Grinberg A., Niehrs C., Morasso M. I. and Westphal H. (2006). Dkk2 plays an essential role in the corneal fate of the ocular surface epithelium. Development 133, 2149-2154. 10.1242/dev.02381 [DOI] [PubMed] [Google Scholar]
- Ng G. Y., Yeh L. K., Zhang Y., Liu H., Feng G. S., Kao W. W. and Liu C. Y. (2013). Role of SH2-containing tryosine phosphatase Shp2 in mouse corneal epithial stratification. Invest. Ophthal. Vis. Sci. 54, 7933-7942. 10.1167/iovs.13-12646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüsslein-Volhard C. and Wieschaus E. (1980). Mutations affecting segment number and polarity in Drosophila. Nature 287, 795-801. 10.1038/287795a0 [DOI] [PubMed] [Google Scholar]
- Perl A.-K. T., Wert S. E., Nagy A., Lobe C. G. and Whitsett J. A. (2002). Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc. Natl. Acad. Sci. USA 99, 10482-10487. 10.1073/pnas.152238499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner A., Hsieh J.-C., Smallwood P. M., Gilbert D. J., Copeland N. G., Jenkins N. A. and Nathans J. (1997). A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc. Natl. Acad. Sci. USA 94, 2859-2863. 10.1073/pnas.94.7.2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi A., Converse R. L., Liu C. Y., Zhou F., Kao C. W. and Kao W. W. (1998). Identification of the cornea-specific keratin 12 promoter by in vivo particle-mediated gene transfer. Invest. Ophthalmol. Vis. Sci. 39, 2554-2561. [PubMed] [Google Scholar]
- Wang R. N., Green J., Wang Z., Deng Y., Qiao M., Peabody M., Zhang Q., Ye J., Yan Z., Denduluri S. et al. (2014). Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 1, 87-105. 10.1016/j.gendis.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. E., Liu J. J. and Mohan R. R. (1999). Stromal-epithelial interactions in the cornea. Prog. Retin. Eye Res. 18, 293-309. 10.1016/S1350-9462(98)00017-2 [DOI] [PubMed] [Google Scholar]
- Woo Y. C., Xu A., Wang Y. and Lam K. S. L. (2013). Fibroblast growth factor 21 as an emerging metabolic regulator: clinical perspectives. Clin. Endocrinol. 78, 489-496. 10.1111/cen.12095 [DOI] [PubMed] [Google Scholar]
- Yang A., Kaghad M., Wang Y., Gillett E., Fleming M. D., Dötsch V., Andrews N. C., Caput D. and McKeon F. (1998). p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol. Cell 2, 305-316. 10.1016/S1097-2765(00)80275-0 [DOI] [PubMed] [Google Scholar]
- Yasunaga M., Oumi N., Osaki M., Kazuki Y., Nakanishi T., Oshimura M. and Sato K. (2011). Establishment and characterization of a transgenic mouse model for in vivo imaging of Bmp4 expression in the pancreas. PLoS ONE 6, e24956 10.1371/journal.pone.0024956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias A. L. and Gage P. J. (2010). Canonical Wnt/β-catenin signaling is required for maintenance but not activation of Pitx2 expression in neural crest during eye development. Dev. Dyn. 239, 3215-3225. 10.1002/dvdy.22459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Call M. K., Yeh L.-K., Liu H., Kochel T., Wang I.-J., Chu P.-H., Taketo M. M., Jester J. V., Kao W. W.-Y. et al. (2010). Aberrant expression of a beta-catenin gain-of-function mutant induces hyperplastic transformation in the mouse cornea. J. Cell Sci. 123, 1285-1294. 10.1242/jcs.063321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Kao W. W.-Y., Pelosi E., Schlessinger D. and Liu C.-Y. (2011). Notch gain of function in mouse periocular mesenchyme downregulates FoxL2 and impairs eyelid levator muscle formation, leading to congenital blepharophimosis. J. Cell Sci. 124, 2561-2572. 10.1242/jcs.085001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Chang W.-H., Fong B., Gao F., Liu C., Al Alam D., Bellusci S. and Lu W. (2014). Regulation of iPS cell induction by Wnt/β-catenin signaling. J. Biol. Chem. 289, 9221-9232. 10.1074/jbc.M113.542845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X.-J., Liu Y., Dai Z.-M., Zhang X., Yang X., Li Y., Qiu M., Fu J., Hsu W., Chen Y. et al. (2014). BMP-FGF signaling axis mediates Wnt-induced epidermal stratification in developing mammalian skin. PLoS Genet. 10, e1004687 10.1371/journal.pgen.1004687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieske J. D. (2004). Corneal development associated with eyelid opening. Int. J. Dev. Biol. 48, 903-911. 10.1387/ijdb.041860jz [DOI] [PubMed] [Google Scholar]