Fig. 1.
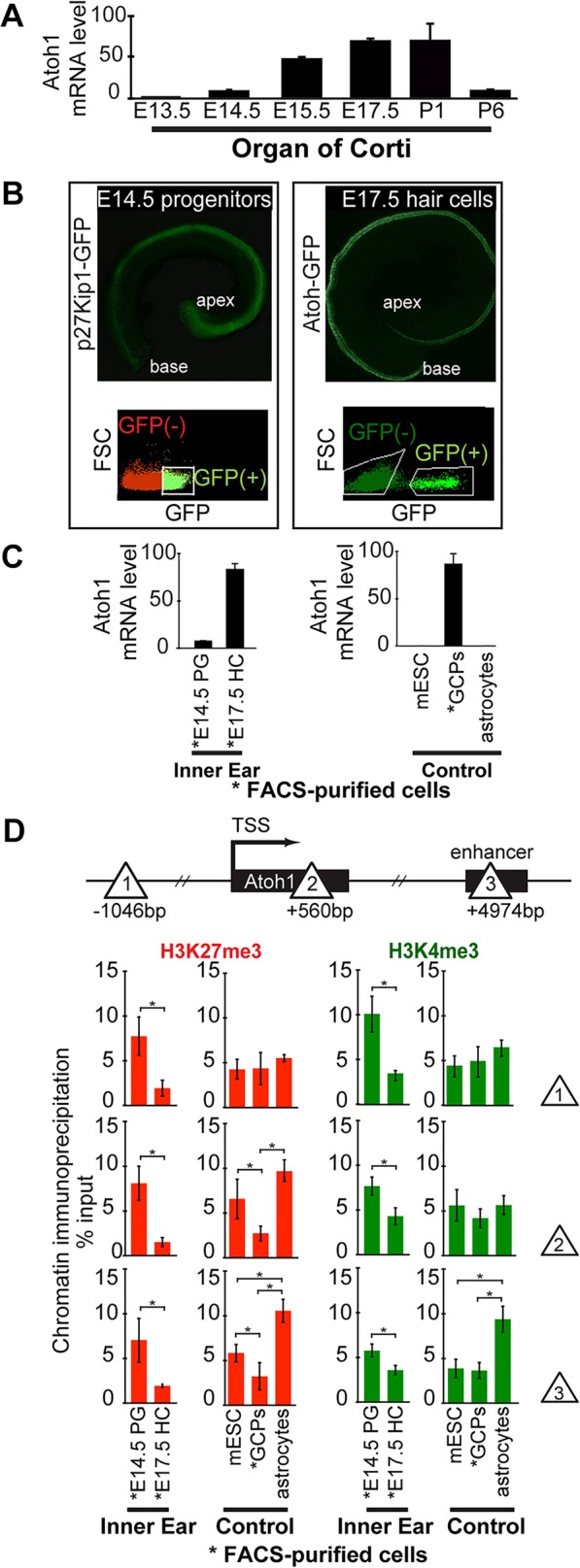
Micro-chromatin immunoprecipitation (µChIP) shows that the Atoh1 gene is bivalent (H3K27me3+ and H3K4me3+) in prosensory progenitors of the organ of Corti, and that H3K27me3 levels are strongly reduced in differentiating hair cells. (A) Relative Atoh1 mRNA levels in the cochlea increase during hair cell differentiation. Levels peak at E17.5 and then decrease during postnatal maturation. Atoh1 mRNA levels extracted from whole cochleae were analyzed at each time point by qPCR. Atoh1 expression levels are normalized to Gapdh as internal reference. Results are mean±s.e.m. (n=3). (B) Representative fluorescence images of whole-mount cochleae and FACS gating used to purify E14.5 progenitors (left, p27Kip1-GFP transgene), and nascent E17.5 hair cells (right, Atoh1-GFP transgene). (C) Relative Atoh1 mRNA levels in FACS-purified progenitors and hair cells, and in control cell types E14.5 progenitor cells (PG), E17.5 hair cells (HC), mouse embryonic stem cells (mESC) and P1 cerebellar granule cell precursors (GCPs). Atoh1 expression levels are normalized to Gapdh as internal reference. Results are mean±s.e.m. (n=3). (D) µChIP analysis of the Atoh1 locus indicates a change in relative H3K27me3 levels between Atoh1-expressing hair cells (FACS-purified from P1 Atoh1-GFP transgenic mice), compared with prosensory progenitors (FACS-purified from P1 p27Kip1-GFP transgenic mice). Schematic shows the locations across the Atoh1 locus (sites 1, 2 and 3; triangles) analyzed by µChIP qPCR for the relative abundance of H3K4me3 and H3K27me3. Results are mean±s.e.m., *P<0.05 (n=3).
