Abstract
Cathepsin K has been shown to exhibit antimicrobial and anti-inflammatory activities in the mouse colon. To further elucidate its role, we used Ctsk−/− mice and demonstrated that the absence of cathepsin K was accompanied by elevated protein levels of related cysteine cathepsins (cathepsins B, L, and X) in the colon. In principle, such changes could result in altered subcellular localization; however, the trafficking of cysteine cathepsins was not affected in the colon of Ctsk−/− mice. However, cathepsin K deficiency affected the extracellular matrix constituents, as higher amounts of collagen IV and laminin were observed. Moreover, the localization pattern of the intercellular junction proteins E-cadherin and occludin was altered in the colon of Ctsk−/− mice, suggesting potential impairment of the barrier function. Thus, we used an ex vivo method for assessing the mucus layers and showed that the absence of cathepsin K had no influence on mucus organization and growth. The data of this study support the notion that cathepsin K contributes to intestinal homeostasis and tissue architecture, but the lack of cathepsin K activity is not expected to affect the mucus-depending barrier functions of the mouse colon. These results are important with regard to oral administration of cathepsin K inhibitors that are currently under investigation in clinical trials.
Keywords: cysteine cathepsins, colon, intercellular junctions, intestinal barrier, mucus
Introduction
Cathepsin K is a cysteine protease first recognized for its high expression in the ovary and osteoclasts (Brömme and Okamoto, 1995). Cathepsin K in osteoclasts is essential for bone remodeling because it exhibits collagenolytic activity and has been shown to degrade type I collagen during bone matrix removal (Gelb et al., 1996; Saftig et al., 1998). This cathepsin has been in the focus of osteoclast-specific therapeutic strategies, aiming at an effective treatment of pathological conditions such as osteoporosis (Deaton and Tavares, 2005; Yasuda et al., 2005; Desmarais et al., 2009; Podgorski, 2009). Apart from osteoclasts, cathepsin K has also been found in other cell types and tissues, such as macrophages (Punturieri et al., 2000), thyroid epithelial cells (Tepel et al., 2000; Jordans et al., 2009; Dauth et al., 2011a), brain parenchyma with neuronal and non-neuronal cells (Dauth et al., 2011b), and cells of the gastrointestinal tract (Haeckel et al., 1999; Mayer et al., 2006). In the human intestine, we observed a brush border localization of cathepsin K, while it was also detected in the mucus of goblet cells (Mayer et al., 2006). This localization suggested secretion of cathepsin K and its re-association with the apical plasma membrane of enterocytes, with potential extracellular functions in the pericellular environment and the intestinal lumen.
Extracellular functions for cathepsin K are conceivable because its proteolytic activity is optimal at acidic pH but still detectable even at neutral to alkaline conditions (Tepel et al., 2000; Jordans et al., 2009). Extracellular cathepsin K has recently been shown to act as an intestinal antibacterial factor with anti-inflammatory potential (Sina et al., 2012).
As cathepsin K inhibitors are already in phase III clinical trials (Lewiecki, 2009; Podgorski, 2009), it is very important to elucidate the roles and functions of this protease in the intestine, which represents one of the major off-target tissues when oral administration routes are aimed. However, treatment of mice with inhibitors of cathepsin D and/or cathepsins B and L resulted in reduced damage of mucosal tissue in a mouse model of colitis (Menzel et al., 2006), demonstrating that protease inhibition during chronic inflammation can also potentially result in beneficial effects.
In most cases of intestinal inflammation, impairment of the barrier function is observed. Intercellular junctions are important players in the maintenance of intestinal tightness and epithelial integrity (Gibson et al., 1995; Hanby et al., 1996; Karayiannakis et al., 1998; Schmitz et al., 1999; Gassler et al., 2001). Therefore, our studies have focused on tight and adherens junction proteins in the colonic epithelium, in order to investigate whether cathepsin K deficiency has an impact on the expression and localization patterns of these proteins. Previous studies of our group have shown that the non-regulated release of cathepsin B after intestinal trauma leads to alterations in intercellular junction proteins (Vreemann et al., 2009), and as cathepsin K is also known to exert extracellular functions in the intestine it was tempting to speculate that its absence will have an impact on junctional complexes.
Another important role of the intestinal barrier in orchestrating the symbiosis of commensal bacteria with the host, meaning the human body, is the intestinal mucus (Backhed et al., 2005; Johansson et al., 2011b). The major component of mucus is the gel-forming protein mucin 2 (Muc2), which is produced and secreted by goblet cells. In the mouse colon, this molecule forms a 50-μm-thick, firmly attached network on top of epithelial cells, which is converted to a three to four times thicker loose outer layer toward the intestinal lumen owing to proteolytic events (Johansson et al., 2008). The inner mucus layer of the colon is well organized and free of bacteria. In contrast, the mucus in the small intestine only consists of a single layer that has similar properties as the outer layer in the colon (Johansson et al., 2008, 2011a). For the structural conversion from the inner to an outer loose layer and the expansion of the Muc2 protein to a wide and loose network, proteolytic events performed by endogenous enzymes are suggested from studies on germ-free mice (Johansson et al., 2008). One example of a pathogen-mediated proteolysis of the Muc2 protein is a cysteine protease secreted by Entamoeba histolytica, which leads to the disruption of the mucus network and thus to the dissolution of the mucus layer (Lidell et al., 2006). Interestingly, cathepsin K was shown to be secreted by human goblet cells (Mayer et al., 2006) and it was also found in the goblet cells and mucus layers of mouse colon (Sina et al., 2012), suggesting a potential involvement of this protease in mucus processing. Therefore, we investigated whether the absence of cathepsin K had influenced the properties of the colon mucus layers; however, no alterations were observed in the patterns of mucus organization and growth of Ctsk−/− mice when compared with wild-type (WT) controls.
In an effort to further investigate the role of this cathepsin in intestinal physiology and homeostasis, here we focused our studies on cathepsin K-deficient mice. The cathepsin K-deficient mice were generated by targeted inactivation of the Ctsk gene by homologous recombination in embryonic stem cells (Saftig et al., 1998). All mice used in our studies were homozygous cathepsin K-deficient males after back-crossing onto the C57BL6/J background (Dauth et al., 2011b). In the present study, we observed alterations in the intestinal proteolytic network, characterized by up-regulation of other cysteine cathepsins such as cathepsins B, L, and X. Moreover, we demonstrated that cathepsin K deficiency has an impact on the extracellular matrix (ECM) constituents in the colon, thereby supporting our previous observations of increased collagen IV in the small and large intestines of Ctsk−/− mice (Dauth et al., 2011a). These findings suggested a role of cathepsin K in ECM remodeling in the mouse intestine, which has also been shown for other cathepsins, namely cathepsins B and L (Vreemann et al., 2009).
Results
Proteolytic network of cysteine cathepsins in the mouse intestine
Misbalance of proteases and their inhibitors can influence intestinal homeostasis and lead to pathological conditions such as inflammation (Medina and Radomski, 2006). In the colon of Ctsk−/− mice, we observed alterations in the proteolytic network due to the absence of cathepsin K protein. More precisely, by immunoblotting analysis, we found that the protein levels of cathepsins B, L, and X (Figure 1A, B, and C, respectively) were significantly elevated in the colon of Ctsk−/− mice when compared with WT controls. The specificity of cathepsins B, L, and X antibodies was confirmed by using tissue from Ctsb−/−, Ctsl−/−, and Ctsz−/− mice, respectively (data not shown). In addition, cryosections prepared from WT and Ctsk−/− mice were stained with cathepsin B-specific antibodies, and this protease (green signals) was localized within vesicles and at the apical plasma membrane of intestinal cells in both WT (Figure 1D, arrows) and Ctsk−/− mice (Figure 1D, arrowheads), without obvious differences in its localization. Similarly, cathepsin L was mainly detectable at the apical plasma membrane and its distribution was not changed by cathepsin K deficiency (Figure 1E, arrows and arrowheads). Cathepsin X was present in a vesicular pattern in both WT and Ctsk−/− colonic tissue (Figure 1F). Thus, translational regulation or altered turnover rates, but not sorting or trafficking, of the cysteine cathepsins B, L, and X were affected by the lack of cathepsin K activity in the mouse colon.
Figure 1.
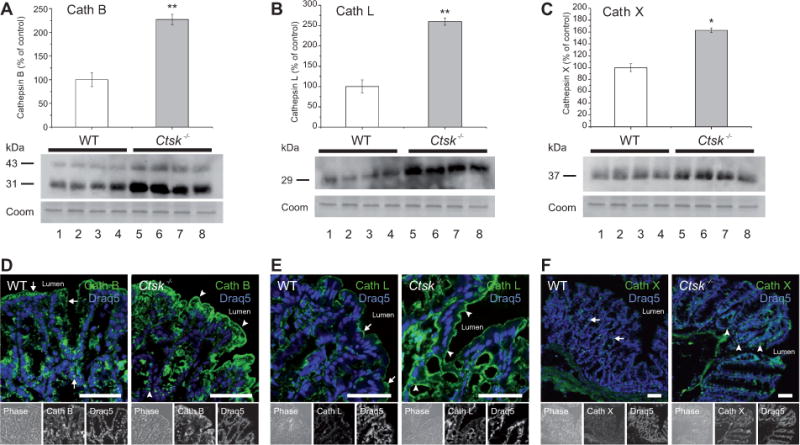
Alterations in the proteolytic network of the Ctsk−/− mouse colon.
(A–C) Densitometry analysis and immunoblots of lysates prepared from the colon of WT (white bars) and Ctsk−/− (gray bars) mice. Protein expression is given as the percentage of WT controls after normalization to total Coomassie-stainable protein as means ± standard error. Note that the protein levels of cathepsin B (A), cathepsin L (B), and cathepsin X (C) were significantly elevated in the colon of Ctsk−/− mice when compared with WT controls. Molecular mass markers are given in the left margin. Levels of significance are indicated as * p < 0.05 and ** p < 0.01. Lanes represent separate individuals. (D–F) Confocal laser scanning micrographs of cryosections prepared from WT and Ctsk−/− mice after staining with cathepsin B-, L-, or X-specific antibodies. Cathepsins B and L (green signals, lower panels, middle) were localized at the apical plasma membrane and in vesicles of intestinal cells in both WT (arrows) and Ctsk−/− mice (arrowheads). Cathepsin X was mainly present in vesicles. Ctsk−/− mice showed stronger immunolabeling, confirming higher cathepsins B, L, and X protein levels. Corresponding phase-contrast micrographs are shown in the lower panels, left. DRAQ5 was used as a nuclear counterstain (blue signals, lower panels, right). Bars, 50 μm.
Absence of cathepsin K has an impact on the ECM of mouse colon
Previous studies of our group have suggested a role of cathepsins B and L in ECM remodeling (Vreemann et al., 2009). As the protein levels of cathepsins B and L were found to be altered in the colon of Ctsk−/− mice (see Figure 1), we wanted to investigate whether this alteration in the proteolytic network would influence ECM constituents such as collagen IV and laminin. Indeed, we found that the protein levels of collagen IV (Figure 2A) were significantly elevated in the colon of Ctsk−/− mice (Figure 2B, gray bars) when compared with WT controls (white bars). These results were also in line with our previous immunofluorescence analysis in both the small and large intestines (Dauth et al., 2011a). Here, we also conducted immunofluorescence analysis (Figure 2C) for laminin (green signals, arrowheads), indicating increased immunostainable levels of this ECM protein in the colonic muscular part of Ctsk−/− mice. These observations suggest a role of cathepsin K in the remodeling of ECM and in the integrity of the basal lamina in the mouse intestine. However, it is still questionable whether cathepsin K regulates the protein levels of ECM constituents through direct or indirect pathways. Although various cysteine cathepsins exhibited higher protein levels in the colon of Ctsk−/− mice, obviously these enzymes could not compensate for the function of cathepsin K in ECM remodeling, thus highlighting its higher efficiency as collagenase and elastase in comparison with cathepsins B, L, and X.
Figure 2.
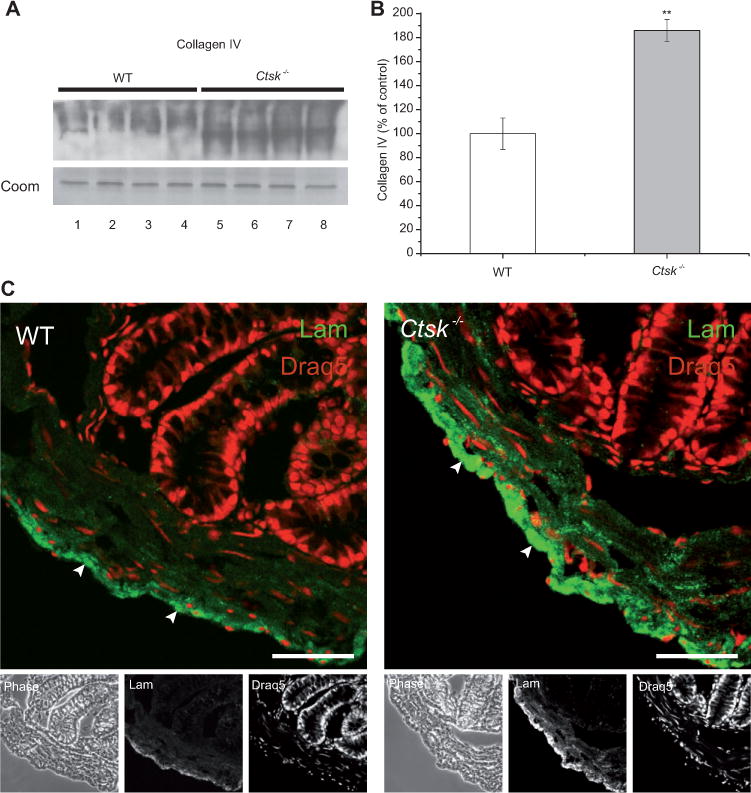
Differences in the ECM of WT and Ctsk−/− mice.
(A) Immunoblot of lysates prepared from the colon of WT (white bars) and Ctsk−/− (gray bars) mice, and (B) densitometry analysis. Protein expression is given as the percentage of WT controls after normalization to total Coomassie-stainable protein as means ± standard error. Note that the protein levels of the ECM constituent collagen IV were significantly elevated in the colon of Ctsk−/− mice when compared with WT controls. Levels of significance are indicated as ** p < 0.01. Lanes represent separate individuals. (C) Confocal laser scanning micro-graphs of cryosections prepared from WT and Ctsk−/− mice after staining with laminin-specific antibodies. Laminin (green signals, lower panels, middle) was present in the colonic ECM (arrowheads) of WT and Ctsk−/− mice. Stronger immunolabeling was observed in the colon of Ctsk−/− mice, indicating higher levels of laminin compared with WT controls. The corresponding phase-contrast micrographs are shown in the lower panels, left. DRAQ5 was used as a nuclear counterstain (red signals, lower panels, right). Bars, 50 μm.
Comparison of intestinal architecture between WT and Ctsk−/− mice
Because the ECM serves as an important structural support, our next step was to study whether the observed alterations in the proteolytic network and the basal lamina of the colon of Ctsk−/− mice had influenced the overall tissue morphology and architecture. We compared cryosections prepared from the colon of WT and Ctsk−/− mice, and no morphological changes were observed regarding the thickness of mucosa, submucosa, and muscle layer (Figure 3 A–C and C′). After staining with the nuclear marker DRAQ5 (Figure 3A′ and B′), we were able to analyze the cell numbers, and we obtained comparable results for the numbers of cells per crypts for both WT and Ctsk−/− mice (Figure 3C″).
Figure 3.
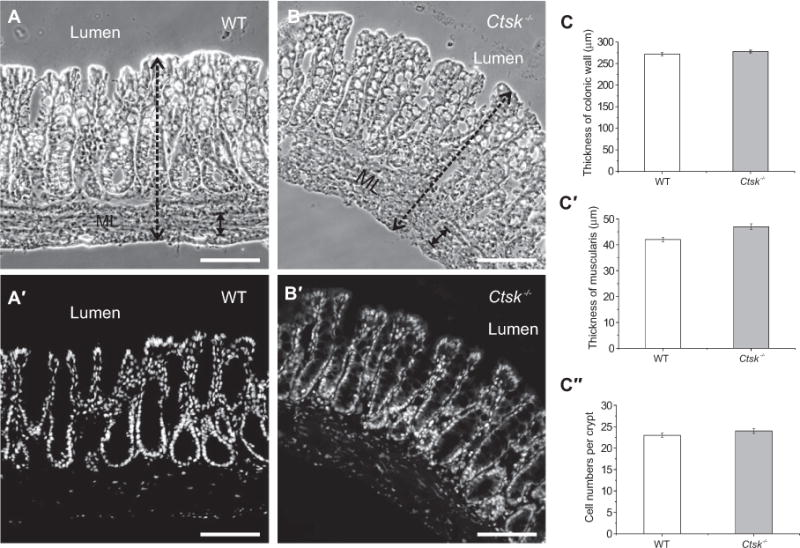
Comparison of intestinal morphology in the colon of WT and Ctsk−/− mice.
Phase-contrast micrographs of cryosections prepared from WT and Ctsk−/− mice (A and B). Confocal laser scanning micrographs of cryosections after staining with the nuclear marker DRAQ5 (A′ and B′). No morphological alterations, such as in the thickness of the mucosa and muscularis layers (ML), or in total cell numbers were observed in the colon of Ctsk−/− mice when compared with WT controls (C, C′, and C″, respectively). Bars, 100 μm.
Cathepsin K-deficient mice display alterations in the expression and localization patterns of intercellular junctions in their colonic epithelium
Intercellular junctions, such as tight and adherens junctions, play a crucial role in the maintenance of intestinal integrity and stability. We initially checked for the expression and localization pattern of the adherens junction protein E-cadherin. By immunoblotting analysis (Figure 4A), we found significantly elevated levels of E-cadherin in the colon of Ctsk−/− mice (Figure 4B, gray bars) when compared with WT controls (white bars). By immunofluorescence microscopy (Figure 4C), we then analyzed the distribution of E-cadherin (green signals, arrowheads) and we observed localization to cell junctions in WT and Ctsk−/− mice. In addition, the stronger immunolabeling of E-cadherin observed in Ctsk−/− mice confirmed the higher levels of this protein in their colonic epithelial cells and more abundant adherens junctions.
Figure 4.
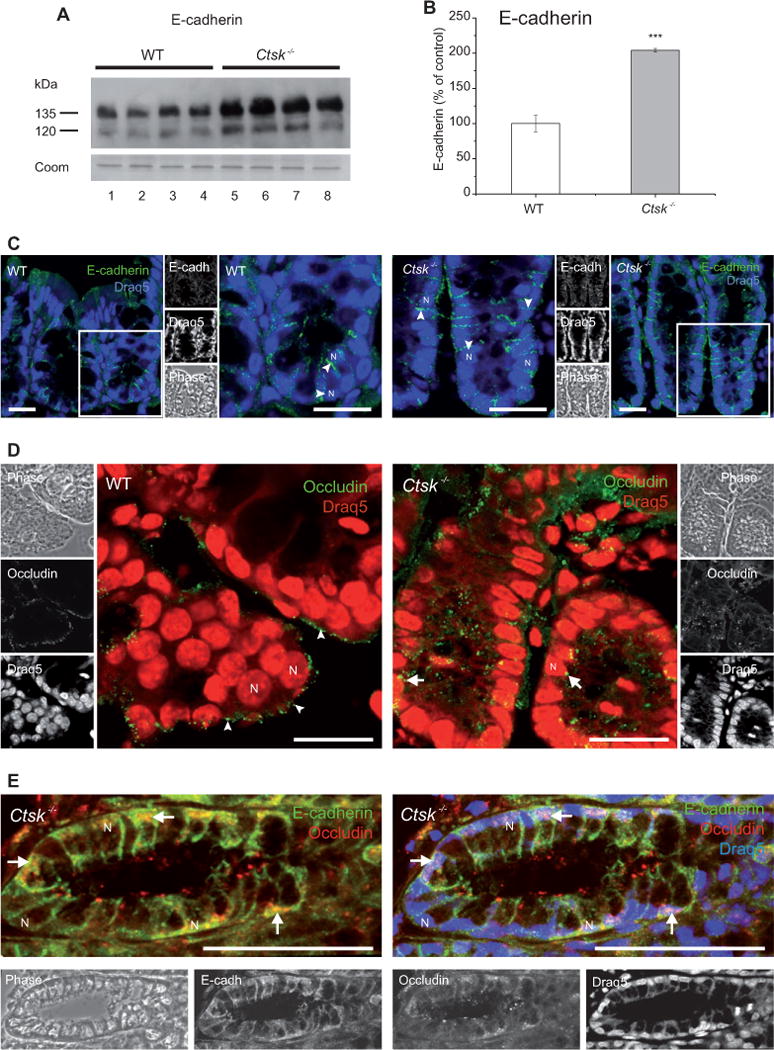
Expression and localization of E-cadherin and occludin in the mouse colon.
(A) Immunoblot of lysates prepared from the colon of WT (white bars) and Ctsk−/− (gray bars) mice, and (B) densitometry analysis. Protein expression is given as the percentage of WT controls after normalization to total Coomassie-stainable protein as means ± standard error. Protein levels of E-cadherin were significantly elevated in the colon of Ctsk−/− mice when compared with WT controls. Molecular mass markers are given in the left margin. Levels of significance are indicated as *** p < 0.001. Lanes represent separate individuals. (C, D) Confocal laser scanning micrographs of cryosections prepared from WT and Ctsk−/− mice after staining with E-cadherin- and occludin-specific antibodies (C and D, respectively). E-cadherin (green signals, side panels, top) exhibited a basolateral localization pattern (arrowheads) in both WT and Ctsk−/− mice. Stronger immunolabeling was observed in the colon of Ctsk−/− mice (C), confirming higher E-cadherin protein levels. Occludin (green signals, side panels, middle) showed a labeling typical for tight junctions at the apical plasma membrane in the colonic epithelium of WT mice (D, arrowheads). This localization pattern was disrupted in Ctsk−/− mice (D, arrows), suggesting alterations in the colonic tight junctions due to cathepsin K deficiency. The notion of dislocalized occludin in Ctsk−/− mice was confirmed by double labeling of adherens and tight junctions (E). Corresponding phase-contrast micrographs are shown in the side panels. DRAQ5 was used as a nuclear counterstain (blue or red signals). N, nucleus; bars, 20 μm (C) and 50 μm (D and E).
We then focused on the multispan tight junction protein occludin. Interestingly, when we stained colon cryosections from WT and Ctsk−/− mice, we observed an altered distribution of occludin (Figure 4D, green signals). More precisely, in WT mice, occludin was detected as expected at the apical poles of the colonic epithelial cells (Figure 4D, arrowheads), whereas in Ctsk−/− mice it was found throughout the cell (Figure 4D, arrows). These results were further confirmed by co-immunolabeling of E-cadherin and occludin (Figure 4E, green and red signals, respectively).
Several studies have demonstrated localization shifts of tight junction proteins, such as occludin and claudin-1, in the chronically inflamed intestine. Thus, in most cases, these alterations have been associated with pathological conditions of the intestine, including inflammatory bowel disease (Boudreau et al., 2007; Noth et al., 2011; Poritz et al., 2011). The integrity of intercellular junctions is not only a sign for the differentiation of the intestinal mucosa, but more so, junctional complexes enable maintaining the functional states of intestinal cells. Our results therefore demonstrated that the functional polarity of intestinal epithelial cells was not affected by the lack of cathepsin K protein.
Cathepsin K deficiency has no impact on the patterns of mucus organization and growth
To investigate the possible role of cathepsin K on the formation or secretion of the mucus layers, we performed immunofluorescence microscopy on cryosections of different parts of the small and large intestines, as well as ex vivo measurements of the mucus in the colon of Ctsk−/− mice. With the latter technique, we determined the increase of the mucus thickness growth in the two mucus layers every 15 min over a time span of 45 min. The secretion rate and the regeneration of the mucus layers in the colon of Ctsk−/− mice were comparable to those in the WT animals (Figure 5A). Accordingly, no significant deviation was detectable for each time point.
Figure 5.
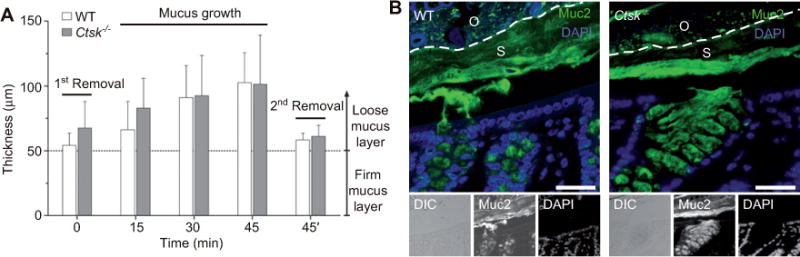
No changes were induced in the colon mucus layers by the absence of cathepsin K.
(A) Mucus growth in the colon of WT and Ctsk−/− mice within 45 min. White bars represent the regeneration of the mucus thickness in WT mice and gray bars in Ctsk−/− mice. t = 0 determines the measurement after the initial removal of the outer mucus layer by aspiration. Likewise, t = 45′ defines the second removal of the outer layer after the measurements. Mucus regrowth was assessed by measuring every 15 min after the first removal (mucus growth). The dotted line corresponds to the inner mucus layer, which is not removable by aspiration. (B) Fluorescence and differential interference contrast (DIC) microscopy of Carnoyfixed sections prepared from the colon of WT and Ctsk−/− mice after staining with a Muc2-specific antiserum. The Muc2 staining reflects the organization of the two colonic mucus layers consisting of one highly stratified layer (S) and an outer layer (O) separated along the dotted line. Both the WT and Ctsk−/− mice showed an intact inner mucus layer. DAPI was used as a nuclear counterstain (blue signals). Both figures show some separation between the epithelium and the inner mucus layer owing to technical reasons. Bars, 50 μm.
Immunostaining analysis of the Muc2 mucin, which is the main glycoprotein and structural compound of the mucus layers, showed a normal inner stratified mucus layer morphology in the Ctsk−/− colon like in WT mice (Figure 5B). In summary, we could not observe any specific phenotype in the composition and assembly of the mucus layers in the colon of Ctsk−/− mice compared with WT mice.
Discussion
Cysteine cathepsins are known to exhibit a variety of functions in diverse tissues, including the small and large intestines. The localization pattern of these proteases differs in the intestine, suggesting both intracellular and extracellular scenes of action (Brix et al., 2008; Arampatzidou et al., 2011a,b). In human enterocytes, cathepsin B was found to reside within endolysosomes, whereas procathepsin L and cathepsin K were detected in association with the apical plasma membrane, and cathepsin K was also abundant in and secreted by goblet cells (Mayer et al., 2006). Cysteine cathepsin-deficient mice, including Ctsk−/− animals, are important in proving further functions of these proteases in the immune system, in the thyroid gland, in adipose tissues, and in the central nervous system besides many more functions in a variety of other cell types and tissues (for reviews, see Brix et al., 2008; Reiser et al., 2010; Dauth et al., 2011a,b).
One of the roles that have been suggested for cathepsins in the mouse intestine is their involvement in the remodeling of the ECM and more precisely in the turnover of collagen IV (Vreemann et al., 2009). The collagenase activity of cathepsin K has also been shown to be vitally important in bone and lung tissue (Gelb et al., 1996; Saftig et al., 1998; Srivastava et al., 2008).
However, cysteine cathepsins beyond and including cathepsin K have been associated with pathological conditions of the intestine, e.g., chronic inflammatory disease (Medina and Radomski, 2006; Menzel et al., 2006). Hence, balance and proper regulation of the proteolytic network is crucial for maintaining intestinal homeostasis and integrity.
Recently, cathepsin K has attracted special attention, as the cathepsin K inhibitor odanacatib, designed for osteoporosis treatment, is in phase III clinical trials (Podgorski, 2009; Rachner et al., 2011; Dauth et al., 2011a). However, previous studies on other cathepsin K inhibitors revealed off-target effects in various tissues, including the central nervous system, kidney, and liver (Desmarais et al., 2008). Although odanacatib exhibits minimal off-target effects owing to its high selectivity for cathepsin K (Gauthier et al., 2008), it is still unknown what the long-term effects of cathepsin K inhibition will be on non-targeted tissues such as the intestine. For instance, it has been shown that the deficiency or pharmacological inhibition of cathepsin K leads to reduced body weight gain and decreased insulin and glucose circulating levels in mice (Yang et al., 2008). Cathepsin K is also known to play a vital role in the liberation of thyroid hormones that are involved in a variety of physiological processes, including regulation of metabolic pathways and body weight (Tepel et al., 2000; Dauth et al., 2011a). Moreover, it has been demonstrated that the intestinal microbiota are altered in cathepsin K-deficient mice and an antimicrobial role has been suggested for cathepsin K (Sina et al., 2012). Thus, it is important to consider the potential consequences that may result from oral drug administration and off-target inhibition of cathepsin K that might rely on both the direct and indirect effects of this protease. Consequently, it is of great interest to elucidate the roles that cathepsin K fulfills in the intestine and to determine potential alterations induced by a lack of this cysteine protease.
Cathepsin K-deficient mice represent a suitable model for this type of studies, and the colon of these mice was used for both biochemical and morphological analyses. We demonstrated that the absence of cathepsin K in the mouse colon leads to alterations in the proteolytic network, with protein levels of specific cathepsins, i.e., cathepsins B, L, and X, being significantly elevated. Our results suggest a compensatory effect among cysteine cathepsins in the colon, which is already known for other tissues. For example, cathepsins K and L are already known to compensate the function of each other in the thyroid (Friedrichs et al., 2003). In addition, it was shown that in the thyroid of Ctsk−/− mice, the distribution and localization of cathepsin L was altered by the absence of cathepsin K. Moreover, previous studies of our group have suggested a role for cathepsins B and L in ECM remodeling. More precisely, we found that non-directed, traumatic release of cysteine cathepsins resulted in massive ECM damage, while cathepsin B- and cathepsin L-deficient mice have significantly increased protein levels of collagen IV in their intestine compared with WT animals (Mayer et al., 2009; Vreemann et al., 2009).
In the present study, we demonstrated that the absence of cathepsin K has an impact on ECM constituents, such as collagen IV and laminin. In the colon of Ctsk−/− mice, the levels of collagen IV and the immunostaining intensities with laminin-specific antibodies were significantly higher than in WT animals, suggesting a role for cathepsin K in the remodeling of ECM and basement membrane. The notion of a major task for cathepsin K in ECM turnover can also be deduced from the severe side effects observed in cathepsin K inhibitor treatments, namely skin rashes and fibrotic scleroderma, eventually resulting in the accumulation of collagen fibers (Desmarais et al., 2008, 2009; Brömme, 2011). The basement membrane plays an important role in maintaining intestinal structure and compartmentalization, while it is also involved in various cellular processes such as adhesion, proliferation, and differentiation (Timpl, 1996; Mahoney et al., 2008). Hence, alterations in the basement membrane components could influence the overall tissue architecture and function. Morphological studies enabled us to verify whether this scenario was true for the Ctsk−/− mice. Our results revealed no differences between WT and Ctsk−/− mice, regarding the colonic epithelial cells, goblet cells, submucosa and muscularis layer, as well as the total cell numbers, in accordance with recent findings (Sina et al., 2012).
A major function of the intestine is its barrier function. Several studies have shown that intercellular junctions contribute in maintaining this intestinal barrier, while impairment of their structure and function has been associated with inflammatory responses (Gibson et al., 1995; Hanby et al., 1996; Karayiannakis et al., 1998; Schmitz et al., 1999; Gassler et al., 2001). Therefore, we analyzed the expression and localization pattern of E-cadherin and occludin. The adherens junction protein E-cadherin has a pivotal role in cell survival, proliferation, and apico-basal polarity (Lien et al., 2006). The absence of cathepsin K resulted in elevated E-cadherin protein levels in the colon of Ctsk−/− mice. Various cathepsins, namely cathepsins B, L, and X, have been shown to act on E-cadherin as a substrate when the proteases are secreted into the extracellular space in tumor conditions (Gocheva et al., 2006). Therefore, the elevated levels of E-cadherin in the colon of Ctsk−/− mice could possibly be explained by the notion that this protein can also be cleaved by cathepsin K. However, the localization of E-cadherin was comparable between WT and Ctsk−/− mice and followed the expected basolateral pattern.
In contrast, the localization pattern of the tight junction protein occludin, serving as molecular fence between the apical and the basolateral plasma membrane domains, was altered in the colon of Ctsk−/− mice. Cathepsin K deficiency resulted in redistribution of occludin away from the tight junctions and toward the cytoplasm. Several studies have demonstrated such localization shifts of tight junction proteins such as occludin and claudin-1, and have associated them with pathological conditions of the intestine (Boudreau et al., 2007; Noth et al., 2011; Poritz et al., 2011). Interestingly, it was shown that inhibition of cathepsin L promotes nuclear localization of claudin-1 in intestinal epithelial cells (Boudreau et al., 2007). Moreover, posttranslational phosphorylation of occludin has also been suggested to mediate the function and localization pattern of this tight junction protein (Paris et al., 2008).
In addition, the integrity of intercellular junctions is also important for proper differentiation and maintenance of the functional state of the intestinal epithelium. In an effort to investigate whether the observed changes regarding tight junctions had influenced intestinal cell differentiation, we studied the localization pattern of aminopeptidase N (APN) (Howell et al., 1993; Mina-Osorio, 2008) in both WT and Ctsk−/− mice. We observed that cathepsin K deficiency induced no alterations in the distribution of this differentiation marker (data not shown).
Another important determinant of intestinal barrier function is the mucus layer that is mainly organized by a single protein, the Muc2 mucin. It has been shown that a cysteine protease from E. histolytica is able to cleave this mucin and disrupt the mucus layers (Lidell et al., 2006). However, the addition of a protease inhibitor cocktail in vivo can inhibit the secretion and thereby the increase of mucus thickness in the rat colon (Johansson et al., 2008). Furthermore, it has been recently described that cathepsin K can be found inside goblet cells and in the mucus layers of both humans and mice (Mayer et al., 2006; Sina et al., 2012). Thus, it has to be co-localized with the Muc2 mucin and could, as an endogenous cysteine protease, likely be involved in mucus layer formation or organization. To address the possible functions of cathepsin K inside the mucus layers, we used a newly established ex vivo method to measure the mucus growth in the colon of Ctsk−/− mice (Gustafsson et al., 2011). With this method, we are able to measure the increase of the mucus in colon explants within a time window of 45 min. The initial (loose) mucus is removed by pipetting (t = 0), whereas the remaining measurable amount resembles the stratified mucus layer. Thus, we focus on the determination of regrowth of newly synthesized mucus layers and data points are taken every 15 min until this mucus is removed again by pipetting after 45 min. In conclusion, the measurements of the colonic mucus layer in the cathepsin K-deficient mice were highly comparable to those in the WT animals. These findings suggest that the absence of cathepsin K does not have an effect on the growth and remodeling of colon mucus layers in the measured time interval of 45 min. In addition, immunofluorescence analysis of Muc2 in colon cryosections of WT and Ctsk−/− mice confirmed the observations from the mucus measurements, as no differences were demonstrated between the two genotypes. Nevertheless, as other cysteine cathepsins were shown to be up-regulated in the colon of Ctsk−/− mice, thus pinpointing to compensatory processes, we cannot completely exclude that cathepsin K is involved in mucus processing. Further research is required to investigate the functions of cathepsin K as well as other proteases in the colonic mucus layers.
In conclusion, our results (see Figure 6) indicated that the tasks of cathepsin K in the turnover of ECM and junctional complex proteins are more important than its extracellular, luminal contributions to intestinal homeostasis. Taking this further, one still has to consider the potential scavenging of orally administered cathepsin K inhibitors by the high amounts of this protease on the luminal side of the epithelium, indicating that high inhibitor dosage might be required for achieving on-target effects. The question of whether it is safe to treat human patients with cathepsin K inhibitors remains open, as the complexity of proteolytic networks makes it even more difficult to predict what the overall outcome will be after such treatment.
Figure 6.
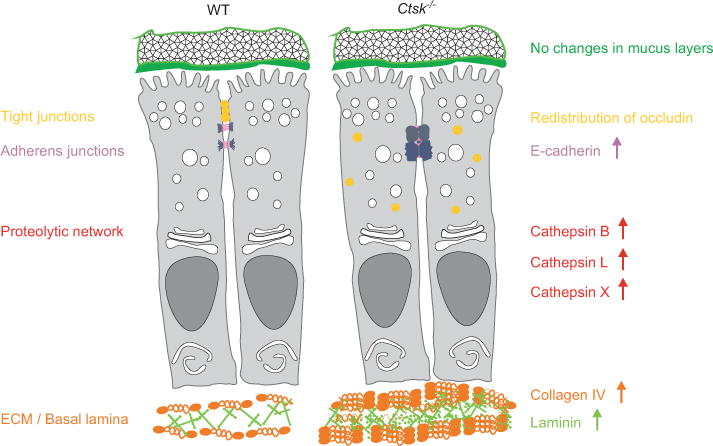
Alterations in the colon of Ctsk−/− mice.
Schematic overview summarizing the alterations observed in the colon of Ctsk−/− mice. Our studies revealed alterations in the proteolytic network of Ctsk−/− mice when compared with WT, characterized by elevated levels of cathepsins B, L, and X. In addition, the absence of cathepsin K had an impact on tight and adherens junctions. More precisely, in the colon of Ctsk−/− mice, we observed redistribution of occludin that was found throughout the cell and not in the tight junctions. Moreover, E-cadherin protein levels were elevated in Ctsk−/− mice, although without changes in its localization pattern. ECM and basal lamina constituents were also influenced by cathepsin K deficiency, as we observed elevated levels of collagen IV and laminin in the colon of Ctsk−/− mice. In addition, the patterns of mucus organization and growth were comparable between WT and Ctsk−/− mice.
Materials and methods
Animals
Cathepsin K-deficient mice (Ctsk−/−) were generated at the University of Göttingen, Germany, and genotyping was performed as described elsewhere (Saftig et al., 1998). All Ctsk−/− and WT C57Bl/6J mice were 6 months old and males. Housing and breeding of animals were conducted in accordance with institutional guidelines and took place in the S1 laboratories of Jacobs University Bremen, Germany, registered as SfAFGJS Az. 513-30-00/2-15-32 and 522-27-11/3-1, 05-A20 and A21. Mice were housed under standard conditions, with a 12 h/12 h light/dark cycle with lights on from 07:00 to 19:00 h, and ad libitum water and food.
Tissue sampling and preparation of tissue extracts
Ctsk−/− and WT mice were anesthetized, the abdominal and thoracic cavities were opened, and the abdominal aorta was cut. For perfusion via the heart, 0.9 % NaCl supplemented with 200 IU heparin (Braun Melsungen AG, Melsungen, Germany) was used. Subsequently, the colon was isolated and washed with an ice-cold 0.9 % NaCl solution. Each colon was divided into two parts. The anterior part was fixed using 4 % paraformaldehyde (PFA) in 200 mm HEPES (pH 7.4) and used for morphological studies, while the posterior part was snap-frozen in liquid nitrogen and used for biochemical analysis. Total tissue extracts were isolated with lysis buffer (PBS containing 0.5 % Triton X-100), and homogenization of the samples was done using a Potter S homogenizer (Sartorius, Göttingen, Germany) at 1000 rpm for 5 min on ice. Homogenates were kept in a rotary mixer for 45 min, while all steps were performed at 4°C. After centrifugation for 10 min at 10 000 × g, supernatants were stored at −20°C. Protein concentration was determined using BSA as a protein standard (Neuhoff et al., 1979).
SDS-PAGE and immunoblotting
Total tissue extracts were normalized to equal amounts of protein (16 μg each), loaded onto 8 % or 12.5 % SDS-polyacrylamide gels, and the separated proteins were then semidry blotted onto nitrocellulose membranes. For the detection of collagen IV, gel electrophoresis and immunoblotting were performed under native conditions (Vreemann et al., 2009), as the collagen IV-specific antibody recognizes only its non-denatured antigen. Blocking was performed overnight at 4 °C using 5 % milk powder in PBS containing 0.3 % Tween-20. After blocking, the following primary antibodies were applied: rabbit anti-mouse collagen IV (Rockland, Philadelphia, PA, USA), goat anti-mouse cathepsin B (Neuromics, through Acris Antibodies, Herford, Germany), goat anti-mouse cathepsin X (R&D Systems, Wiesbaden, Germany), goat anti-mouse cathepsin L (Neuromics), and rabbit anti-human E-cadherin (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). Membranes were subsequently washed and incubated for 1 h at room temperature with HRP-conjugated antibodies. The secondary antibodies used were goat anti-rabbit and rabbit anti-goat (both from Southern Biotech, Birmingham, AL, USA). Immunoreactions were visualized using an enhanced chemiluminescence substrate on CL-XPosure film (both from Pierce through Perbio Science Europe, Bonn, Germany).
Densitometry and statistical analysis
Densitometry analysis of immunoblots was performed using TINA soft ware version 2.09d (Raytest Isotopen-Messgeräte GmbH, Straubenhardt, Germany). Background intensity was subtracted, and all measured optical density/mm2 values were given as mean of intensities per area. The results were expressed as percentage of protein expression in WT controls using Coomassie-stained protein per lane for normalization to account for differences between independent experiments. The two-tailed Student’s t-test of Origin 7.0 SRO (OriginLab Corp., Northampton, MA, USA) was used in order to assess differences between Ctsk−/− and WT mice and determine levels of significance. All data are shown as mean ± standard error of the mean.
Cryosectioning and immunolabeling
After fixation with 4 % PFA in 200 mM HEPES, colon tissue was washed and samples were incubated overnight in 200 mM HEPES buffer (pH 7.4). From each colon sample, pieces of approximately 1-cm length were cut and incubated in Tissue Freezing Medium (Jung, through Leica Microsystems, Nussloch, Germany). After overnight incubation, samples were frozen on dry ice and cryosections of 5 μm were prepared using a Leica CM1900 cryostat (Leica Microsystems). Cryosections were mounted on microscope slides, blocked with 3 % BSA in calcium-magnesium-free (CMF) PBS, and immunolabeled with primary antibodies. Specific primary antibodies were rabbit anti-human cathepsin L (RD Laboratories, Diessen, Germany), goat anti-human laminin (Santa Cruz), rabbit anti-human E-cadherin (Santa Cruz), and goat anti-human occludin (Santa Cruz). After several washing steps with 0.1% BSA in CMF-PBS, sections were incubated with secondary antibodies for 1 h at 37°C. Alexa 488- or Alexa 546-coupled goat anti-rabbit IgG, Alexa 546 donkey anti-goat IgG, and Alexa 488 rabbit anti-goat IgG secondary antibodies were used (all from Invitrogen through Molecular Probes, Karlsruhe, Germany). Double labeling of E-cadherin and occludin was performed by application of the respective primary and secondary antibodies in a consecutive manner. DRAQ5 (Biostatus Limited, Shepshed, Leicestershire, UK) was applied together with the secondary antibodies and served as a nuclear counterstain. Negative controls were prepared in which the specific primary antibodies were omitted and sections were incubated only with secondary antibodies and DRAQ5.
Immunohistostaining of Muc2
For immunostaining, segments of the distal colon (without flushing the luminal content) were fixed in Carnoy’s fixative consisting of 60% ethanol, 30% chloroform, and 10% glacial acetic acid. For sectioning, these were embedded in paraffin, later dewaxed for two times 10-min incubations at 60°C in xylene substitute (Sigma-Aldrich), and hydrated by a decreasing alcohol series from 100% to 30%. Antigen retrieval was achieved by boiling the sections in a solution of 10 Mm sodium citrate and 0.05% Tween-20 (pH 6.0) for 5 min. Before blocking with 5% FCS in PBS for 20 min, the sections were allowed to cool to room temperature. The primary antibody against human Muc2 was incubated overnight at 4°C, and Alexa488 anti-rabbit IgG was used as a secondary antibody together with DAPI (1:30 000, Sigma) for visualizing the nuclei. The fluorescence pictures were taken with a Nikon Eclipse E1000 (Nikon Corp., Tokyo, Japan).
Microscopy
Immunolabeled colon sections were viewed with a Zeiss LSM 510 META laser scanning microscope equipped with argon and helium-neon lasers (Carl Zeiss GmbH, Jena, Germany). Optical sections were obtained with a pinhole setting of 1 Airy unit and at a resolution of 1024 × 1024 pixels, and were further analyzed using LSM 510 soft ware, release 3.2 (Carl Zeiss).
Morphometry
The extensions of the mucosal and muscularis layers in the colon were determined using the measurement tool of the LSM software. Enumeration of cells per crypts was conducted manually. In all cases, at least 25 sections were chosen in an arbitrary fashion from three different WT and Ctsk−/− mice.
Mucus measurements
Mucus measurements in Ctsk−/− and WT mice have been performed as previously described (Gustafsson et al., 2011). The colon was dissected from the mice, opened along the mesenteric border, and mounted between two acrylic plates in an Ussing-type chamber after the removal of the longitudinal muscle layer. Inside the basal chamber, oxygenized Krebs solution (136 mM NaCl, 4. mM KCl, 1.4 mM MgSO4, 1.6 mM KH2PO4, 1.5 mM CaCl2, 27 mM NaHCO3, 10 mM D-glucose, pH 7.4) was applied with a constant perfusion of 5 ml/min (Pump33; Harvard Apparatus, Holliston, MA, USA). The luminal side of the colon sample was directed toward the apical, i.e., upper chamber with a fixed surface diameter of 2.5 mm and covered with 150 μl of Krebs solution, but completed with 10 mM D-mannitol instead of 10 mM D-glucose. The whole chamber system was heated to 37°C (dual-channel heater TC-344B; Warner Instrument Corporation, Hamden, CT, USA).
By the addition of charcoal particles to the Krebs mannitol solution in the upper chamber, the usually transparent mucus was visualized under a stereomicroscope (Leica MZ75; Leica, Wetzlar, Germany). The actual measuring process was performed with a glass micropipette (diameter 5–10 μm), fixed in an angle of 45°. Attached to a micromanipulator with a connected digimatic indicator (Mitutoyo, Kawasaki, Japan), the micropipette was used to measure the distance between the epithelial surface and the charcoal particles on top of the mucus layer. To calculate the average mucus thickness, each measurement comprised five data points on both the mucus layer and the epithelial surface. From these data, the actual vertical mucus thickness was calculated by multiplying the measured distance with the cosinus of 45.
After the initial measurement, the mucus was removed with a pipette adjusted to a fixed volume of 150 μl. To obtain the mucus layer regeneration over 60 min, the measurements were repeated every 15 min. After 45 min, the mucus layer was removed again and the thickness of the remaining mucus was determined.
Acknowledgments
This study was supported by Jacobs University Bremen, project 2140/90140 to KBr, and by the Deutsche Forschungsgemeinschaft (DFG), grant BR 1308/10-1 to KBr. This work was also supported by the Swedish Research Council (no. 7461, 21027), the Swedish Cancer Foundation, the Knut and Alice Wallenberg Foundation, the IngaBritt and Arne Lundberg Foundation, Wilhelm and Martina Lundgren’s Foundation, Torsten och Ragnar Söderbergs Stiftelser, and the Swedish Foundation for Strategic Research-The Mucus-Bacteria-Colitis Center (MBC) of the Innate Immunity Program to AS and GH. The authors would like to thank MSc Sanja Mehandziska and Tripti Tamhane (both from Jacobs University Bremen, Germany) for their contributions during the initial and final phases of the project.
Contributor Information
Maria Arampatzidou, School of Engineering and Science, Research Center MOLIFE – Molecular Life Science, Jacobs University Bremen, Campus Ring 6, D-28759 Bremen, Germany.
André Schütte, Department of Medical Biochemistry, University of Gothenburg, Medicinaregatan 9A, S-41390 Gothenburg, Sweden.
Gunnar C. Hansson, Department of Medical Biochemistry, University of Gothenburg, Medicinaregatan 9A, S-41390 Gothenburg, Sweden
Paul Saftig, Institute of Biochemistry, Christian-Albrechts Universität zu Kiel, Olshausenstraße 40, D-24118 Kiel, Germany.
Klaudia Brix, School of Engineering and Science, Research Center MOLIFE – Molecular Life Science, Jacobs University Bremen, Campus Ring 6, D-28759 Bremen, Germany.
References
- Arampatzidou M, Mayer K, Lolyeva ME, Asrat SG, Ravichandran M, Gunther T, Schule R, Reinheckel T, Brix K. Studies of intestinal morphology and cathepsin B expression in a transgenic mouse aiming at intestine-specific expression of Cath B-EGFP. Biol Chem. 2011a;392:983–993. doi: 10.1515/BC.2011.096. [DOI] [PubMed] [Google Scholar]
- Arampatzidou M, Rehders M, Dauth S, Yu DM, Tedelind S, Brix K. Imaging of protease functions – current guide to spotting cysteine cathepsins in classical and novel scenes of action in mammalian epithelial cells and tissues. Ital J Anat Embryol. 2011b;116:1–19. [PubMed] [Google Scholar]
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- Boudreau F, Lussier CR, Mongrain S, Darsigny M, Drouin JL, Doyon G, Suh ER, Beaulieu JF, Rivard N, Perreault N. Loss of cathepsin L activity promotes claudin-1 overexpression and intestinal neoplasia. Faseb J. 2007;21:3853–3865. doi: 10.1096/fj.07-8113com. [DOI] [PubMed] [Google Scholar]
- Brix K, Dunkhorst A, Mayer K, Jordans S. Cysteine cathepsins: cellular roadmap to different functions. Biochimie. 2008;90:194–207. doi: 10.1016/j.biochi.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Brömme D. Cysteine cathepsins and the skeleton. Clin Rev Bone Miner Metab. 2011;9:83–93. [Google Scholar]
- Brömme D, Okamoto K. Human cathepsin O2, a novel cysteine protease highly expressed in osteoclastomas and ovary molecular cloning, sequencing and tissue distribution. Biol Chem Hoppe Seyler. 1995;376:379–384. doi: 10.1515/bchm3.1995.376.6.379. [DOI] [PubMed] [Google Scholar]
- Dauth S, Arampatzidou M, Rehders M, Yu D, Führer D, Brix K. Thyroid cathepsin K – roles in physiology and thyroid disease. Clin Rev Bone Miner Metab. 2011a;9:94–106. [Google Scholar]
- Dauth S, Sirbulescu RF, Jordans S, Rehders M, Avena L, Oswald J, Lerchl A, Saftig P, Brix K. Cathepsin K deficiency in mice induces structural and metabolic changes in the central nervous system that are associated with learning and memory deficits. BMC Neurosci. 2011b;12:74. doi: 10.1186/1471-2202-12-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaton DN, Tavares FX. Design of cathepsin K inhibitors for osteoporosis. Curr Top Med Chem. 2005;5:1639–1675. doi: 10.2174/156802605775009676. [DOI] [PubMed] [Google Scholar]
- Desmarais S, Black WC, Oballa R, Lamontagne S, Riendeau D, Tawa P, Duong le T, Pickarski M, Percival MD. Effect of cathepsin K inhibitor basicity on in vivo off-target activities. Mol Pharmacol. 2008;73:147–156. doi: 10.1124/mol.107.039511. [DOI] [PubMed] [Google Scholar]
- Desmarais S, Masse F, Percival MD. Pharmacological inhibitors to identify roles of cathepsin K in cell-based studies: a comparison of available tools. Biol Chem. 2009;390:941–948. doi: 10.1515/BC.2009.092. [DOI] [PubMed] [Google Scholar]
- Friedrichs B, Tepel C, Reinheckel T, Deussing J, von Figura K, Herzog V, Peters C, Saftig P, Brix K. Thyroid functions of mouse cathepsins B, K, and L. J Clin Invest. 2003;111:1733–1745. doi: 10.1172/JCI15990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassler N, Rohr C, Schneider A, Kartenbeck J, Bach A, Obermuller N, Otto HF, Autschbach F. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol Gastrointest Liver Physiol. 2001;281:G216–G228. doi: 10.1152/ajpgi.2001.281.1.G216. [DOI] [PubMed] [Google Scholar]
- Gauthier JY, Chauret N, Cromlish W, Desmarais S, Duong le T, Falgueyret JP, Kimmel DB, Lamontagne S, Leger S, LeRiche T, et al. The discovery of odanacatib (MK-0822), a selective inhibitor of cathepsin K. Bioorg Med Chem Lett. 2008;18:923–928. doi: 10.1016/j.bmcl.2007.12.047. [DOI] [PubMed] [Google Scholar]
- Gelb BD, Shi GP, Chapman HA, Desnick RJ. Pycnodysostosis, a lysosomal disease caused by cathepsin K deficiency. Science. 1996;273:1236–1238. doi: 10.1126/science.273.5279.1236. [DOI] [PubMed] [Google Scholar]
- Gibson P, Rosella O, Nov R, Young G. Colonic epithelium is diffusely abnormal in ulcerative colitis and colorectal cancer. Gut. 1995;36:857–863. doi: 10.1136/gut.36.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocheva V, Zeng W, Ke D, Klimstra D, Reinheckel T, Peters C, Hanahan D, Joyce JA. Distinct roles for cysteine cathepsin genes in multistage tumorigenesis. Genes Dev. 2006;20:543–556. doi: 10.1101/gad.1407406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson JK, Ermund A, Johansson ME, Schutte A, Hansson GC, Sjovall H. An ex vivo method for studying mucus formation, properties, and thickness in human colonic biopsies and mouse small and large intestinal explants. Am J Physiol Gastrointest Liver Physiol. 2011;302:G430–G438. doi: 10.1152/ajpgi.00405.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeckel C, Krueger S, Buehling F, Broemme D, Franke K, Schuetze A, Roese I, Roessner A. Expression of cathepsin K in the human embryo and fetus. Dev Dyn. 1999;216:89–95. doi: 10.1002/(SICI)1097-0177(199910)216:2<89::AID-DVDY1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Hanby AM, Chinery R, Poulsom R, Playford RJ, Pignatelli M. Downregulation of E-cadherin in the reparative epithelium of the human gastrointestinal tract. Am J Pathol. 1996;148:723–729. [PMC free article] [PubMed] [Google Scholar]
- Howell S, Brewis IA, Hooper NM, Kenny AJ, Turner AJ. Mosaic expression of membrane peptidases by confluent cultures of Caco-2 cells. FEBS Lett. 1993;317:109–112. doi: 10.1016/0014-5793(93)81502-q. [DOI] [PubMed] [Google Scholar]
- Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson ME, Ambort D, Pelaseyed T, Schutte A, Gustafsson JK, Ermund A, Subramani DB, Holmen-Larsson JM, Thomsson KA, Bergstrom JH, et al. Composition and functional role of the mucus layers in the intestine. Cell Mol Life Sci. 2011a;68:3635–3641. doi: 10.1007/s00018-011-0822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson ME, Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci USA. 2011b;108(Suppl 1):4659–4665. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordans S, Jenko-Kokalj S, Kuhl NM, Tedelind S, Sendt W, Brömme D, Turk D, Brix K. Monitoring compartment-specific substrate cleavage by cathepsins B, K, L, and S at physiological pH and redox conditions. BMC Biochem. 2009;10:23. doi: 10.1186/1471-2091-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayiannakis AJ, Syrigos KN, Efstathiou J, Valizadeh A, Noda M, Playford RJ, Kmiot W, Pignatelli M. Expression of catenins and E-cadherin during epithelial restitution in inflammatory bowel disease. J Pathol. 1998;185:413–418. doi: 10.1002/(SICI)1096-9896(199808)185:4<413::AID-PATH125>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Lewiecki EM. Odanacatib, a cathepsin K inhibitor for the treatment of osteoporosis and other skeletal disorders associated with excessive bone remodeling. IDrugs. 2009;12:799–809. [PubMed] [Google Scholar]
- Lidell ME, Moncada DM, Chadee K, Hansson GC. Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal domain and dissolve the protective colonic mucus gel. Proc Natl Acad Sci USA. 2006;103:9298–9303. doi: 10.1073/pnas.0600623103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien WH, Klezovitch O, Vasioukhin V. Cadherin-catenin proteins in vertebrate development. Curr Opin Cell Biol. 2006;18:499–506. doi: 10.1016/j.ceb.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Mahoney ZX, Stappenbeck TS, Miner JH. Laminin α5 influences the architecture of the mouse small intestine mucosa. J Cell Sci. 2008;121:2493–2502. doi: 10.1242/jcs.025528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer K, Schwartz S, Lentze MJ, Kalff JC, Brix K. Extracellular localization of intestinal cathepsins: implications of their actions during post-operative ileus. In: Vollmar B, editor. XLI Congress of the European Society for Surgical Research. Rostock, Germany: Medimond International Proceedings; 2006. pp. 63–66. [Google Scholar]
- Mayer K, Vreemann A, Qu H, Brix K. Release of endo-lysosomal cathepsins B, D, and L from IEC6 cells in a cell culture model mimicking intestinal manipulation. Biol Chem. 2009;390:471–480. doi: 10.1515/BC.2009.047. [DOI] [PubMed] [Google Scholar]
- Medina C, Radomski MW. Role of matrix metalloproteinases in intestinal inflammation. J Pharmacol Exp Ther. 2006;318:933–938. doi: 10.1124/jpet.106.103465. [DOI] [PubMed] [Google Scholar]
- Menzel K, Hausmann M, Obermeier F, Schreiter K, Dunger N, Bataille F, Falk W, Scholmerich J, Herfarth H, Rogler G. Cathepsins B, L and D in inflammatory bowel disease macrophages and potential therapeutic effects of cathepsin inhibition in vivo. Clin Exp Immunol. 2006;146:169–180. doi: 10.1111/j.1365-2249.2006.03188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mina-Osorio P. The moonlighting enzyme CD13: old and new functions to target. Trends Mol Med. 2008;14:361–371. doi: 10.1016/j.molmed.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhoff V, Philipp K, Zimmer HG, Mesecke S. A simple, versatile, sensitive and volume-independent method for quantitative protein determination which is independent of other external influences. Hoppe Seylers Z Physiol Chem. 1979;360:1657–1670. doi: 10.1515/bchm2.1979.360.2.1657. [DOI] [PubMed] [Google Scholar]
- Noth R, Lange-Grumfeld J, Stuber E, Kruse ML, Ellrichmann M, Hasler R, Hampe J, Bewig B, Rosenstiel P, Schreiber S, et al. Increased intestinal permeability and tight junction disruption by altered expression and localization of occludin in a murine graft versus host disease model. BMC Gastroenterol. 2011;11:109. doi: 10.1186/1471-230X-11-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris L, Tonutti L, Vannini C, Bazzoni G. Structural organization of the tight junctions. Biochim Biophys Acta. 2008;1778:646–659. doi: 10.1016/j.bbamem.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Podgorski I. Future of anticathepsin K drugs: dual therapy for skeletal disease and atherosclerosis? Future Med Chem. 2009;1:21–34. doi: 10.4155/fmc.09.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poritz LS, Harris LR, 3rd, Kelly AA, Koltun WA. Increase in the tight junction protein claudin-1 in intestinal inflammation. Dig Dis Sci. 2011;56:2802–2809. doi: 10.1007/s10620-011-1688-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punturieri A, Filippov S, Allen E, Caras I, Murray R, Reddy V, Weiss SJ. Regulation of elastinolytic cysteine proteinase activity in normal and cathepsin K-deficient human macrophages. J Exp Med. 2000;192:789–799. doi: 10.1084/jem.192.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser J, Adair B, Reinheckel T. Specialized roles for cysteine cathepsins in health and disease. J Clin Invest. 2010;120:3421–3431. doi: 10.1172/JCI42918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saftig P, Hunziker E, Wehmeyer O, Jones S, Boyde A, Rommerskirch W, Moritz JD, Schu P, von Figura K. Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc Natl Acad Sci USA. 1998;95:13453–13458. doi: 10.1073/pnas.95.23.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Bentzel CJ, Riecken EO, Schulzke JD. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999;116:301–309. doi: 10.1016/s0016-5085(99)70126-5. [DOI] [PubMed] [Google Scholar]
- Sina C, Lipinski S, Gavrilova O, Aden K, Rehman A, Till A, Rittger A, Podschun R, Meyer-Hoffert U, Haesler R, et al. Extracellular cathepsin K exerts antimicrobial activity and is protective against chronic intestinal inflammation in mice. Gut. 2012 doi: 10.1136/gutjnl-2011-300076. [DOI] [PubMed] [Google Scholar]
- Srivastava M, Steinwede K, Kiviranta R, Morko J, Hoymann HG, Langer F, Buhling F, Welte T, Maus UA. Overexpression of cathepsin K in mice decreases collagen deposition and lung resistance in response to bleomycin-induced pulmonary fibrosis. Respir Res. 2008;9:54. doi: 10.1186/1465-9921-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepel C, Brömme D, Herzog V, Brix K. Cathepsin K in thyroid epithelial cells: sequence, localization and possible function in extracellular proteolysis of thyroglobulin. J Cell Sci. 2000;113(Pt 24):4487–4498. doi: 10.1242/jcs.113.24.4487. [DOI] [PubMed] [Google Scholar]
- Timpl R. Macromolecular organization of basement membranes. Curr Opin Cell Biol. 1996;8:618–624. doi: 10.1016/s0955-0674(96)80102-5. [DOI] [PubMed] [Google Scholar]
- Vreemann A, Qu H, Mayer K, Andersen LB, Stefana MI, Wehner S, Lysson M, Farcas AM, Peters C, Reinheckel T, et al. Cathepsin B release from rodent intestine mucosa due to mechanical injury results in extracellular matrix damage in early post-traumatic phases. Biol Chem. 2009;390:481–492. doi: 10.1515/BC.2009.055. [DOI] [PubMed] [Google Scholar]
- Yang M, Sun J, Zhang T, Liu J, Zhang J, Shi MA, Darakhshan F, Guerre-Millo M, Clement K, Gelb BD, et al. Deficiency and inhibition of cathepsin K reduce body weight gain and increase glucose metabolism in mice. Arterioscler Thromb Vasc Biol. 2008;28:2202–2208. doi: 10.1161/ATVBAHA.108.172320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda Y, Kaleta J, Brömme D. The role of cathepsins in osteoporosis and arthritis: rationale for the design of new therapeutics. Adv Drug Deliv Rev. 2005;57:973–993. doi: 10.1016/j.addr.2004.12.013. [DOI] [PubMed] [Google Scholar]


