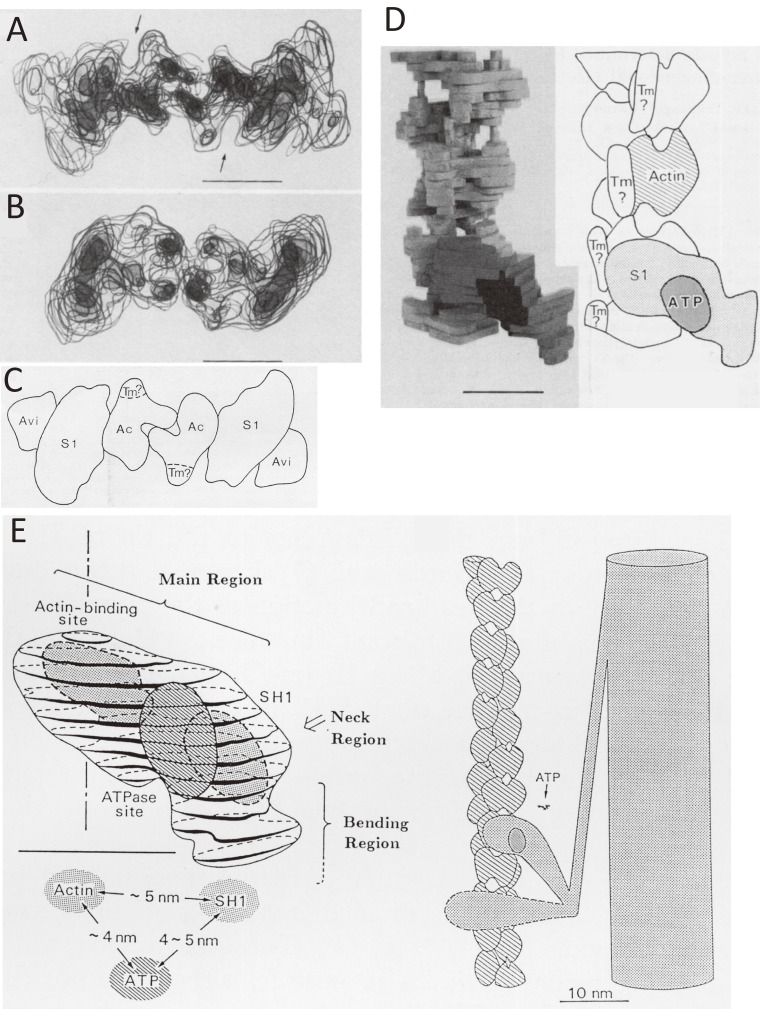
Figure 6.
Location of ATPase site of myosin and its relation to the actin-binding site as revealed by electron microscopy of negatively stained specimens by assuming the helical symmetry of specimens.55) A. The end-on view of the transparent model of actin-tropomyosin-myosin S1-ADP-Vi and avidin. A protrusion on the outer surface of myosin S1 corresponds to avidin. B. The end-on view of actin-tropomyosin-myosin S1 without avidin. C. The schematic illustration of A. D. The discontinuous column-like structures, interpreted as a candidate for tropomyosin in an R-state or an “open” state (right panel of Fig. 15) observed near the grooves of actin double helix. E. The spatial relationship between the actin-binding site, the ATPase site, and reactive thiol (SH1, Cys-707) determined by the developed avidin-biotin system is shown in the left panel.53–55) In the right panel, two myosin heads are depicted differently. One is more bent than the other. The ATP-induced changes of the angle of the bending region against the main region were shown experimentally.64–71)