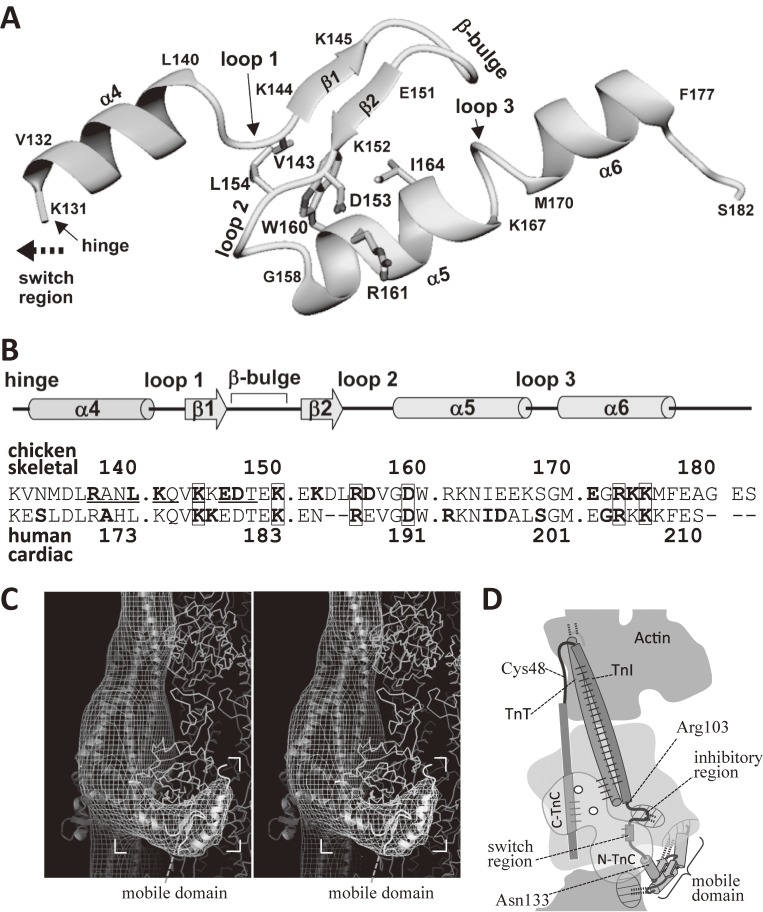
Figure 9.
The atomic structure of the mobile domain of troponin as revealed by NMR spectroscopy of the ternary complex of TnT2, TnI and TnC.102) A. The atomic structure of the mobile domain (residues 131–182 of TnI) in the ternary complex. Figure 7C shows the same structure in color. B. Summary of secondary-structure elements, the sequence of the mobile domain, and that of the corresponding region of the human cardiac muscle. The residues that showed Ca2+-induced changes in chemical shifts are underlined. The residues that interact with actin are shown in bold letters. The numbering shown in the upper line is for skeletal troponin-I. The numbering in the lower line is for the human cardiac one. The residues related to human cardiomyopathy106) are shown in bold letters, and are enclosed with boxes, if they were also involved in the interaction with actin. C. A stereo pair to show the mobile domain (in ribbon format) docked into the image of the troponin arm (net of contour lines). Atomic structure50) of actin is shown in wire format. The residues found to interact with actin were shown in bold letters in the upper line of the panel B. The interaction was mainly electrostatic. D. Summary of the general relation of actin with troponin at low Ca2+ concentrations. The mobile domain of TnI, together with the inhibitory region of TnI, holds the COOH-terminal region of actin. The dotted lines indicate the interactions between TnI and actin. The solid lines indicate the interactions within troponin.