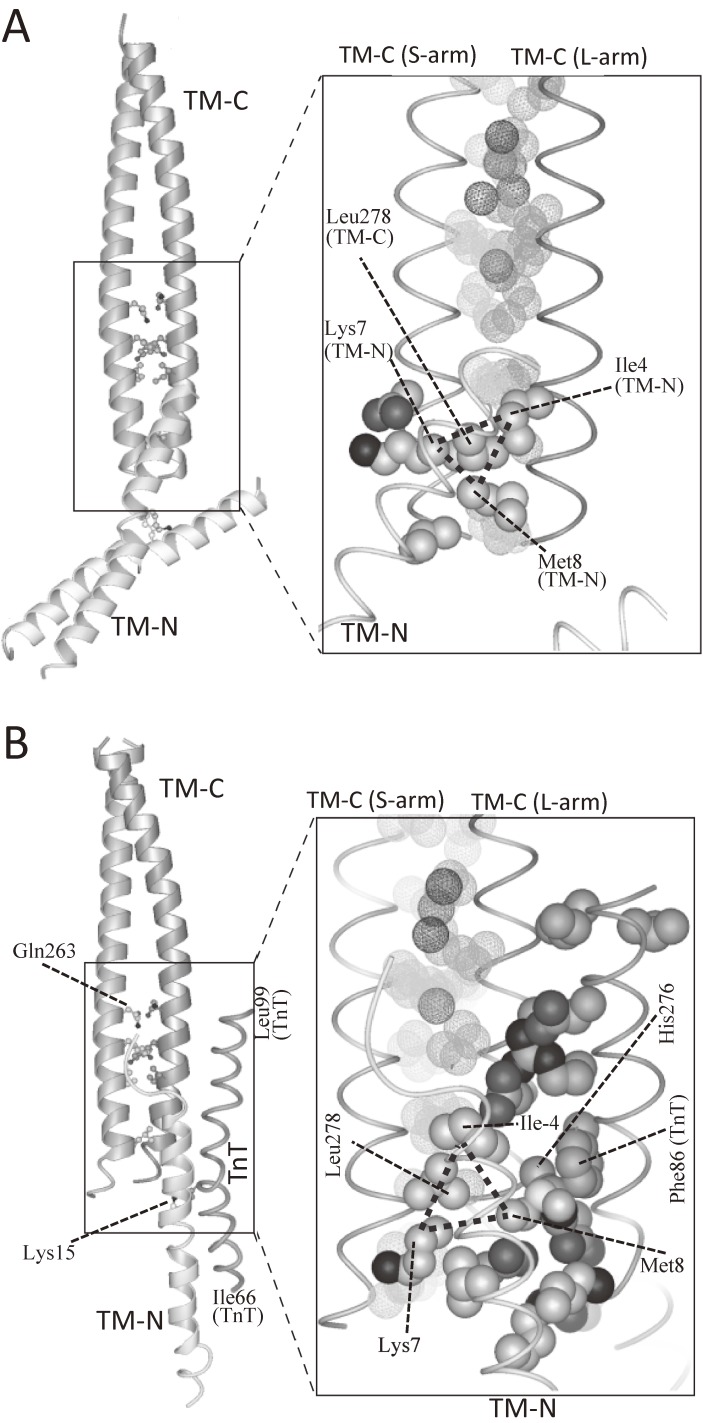
Figure 12.
The atomic structure of tropomyosin junction as determined by X-ray crystallography.119) A. TM-C:TM-N binary complex. B. TM-C:TM-N:TnT ternary complex. In both complexes, responsible residues for the formation of tropomyosin junction were the same: Leu-278, Ile-4, Lys-7, and Met-8. The Leu-278 functions as a “knob”, which binds to a “hole” formed by Ile-4, Lys-7, and Met-8. The “hole” is indicated by a dotted triangle. The interaction between the “knob” and the “hole” was mainly hydrophobic, and the “knob-in-hole” interaction may work as a “ball-and-socket” joint, in which the ball-shaped “knob” moves smoothly inside a curved hollow “hole”. Thus, the “knob-in-hole” interaction may work as an adjustable swivel, which allows the swing of TM-N against TM-C at the junction, and allows to bridge the ∼20-Å gap between the azimuthal position of the COOH-terminal one-third and that of the NH-terminal one-half of tropomyosin at low Ca2+ concentrations.