Figure 15.
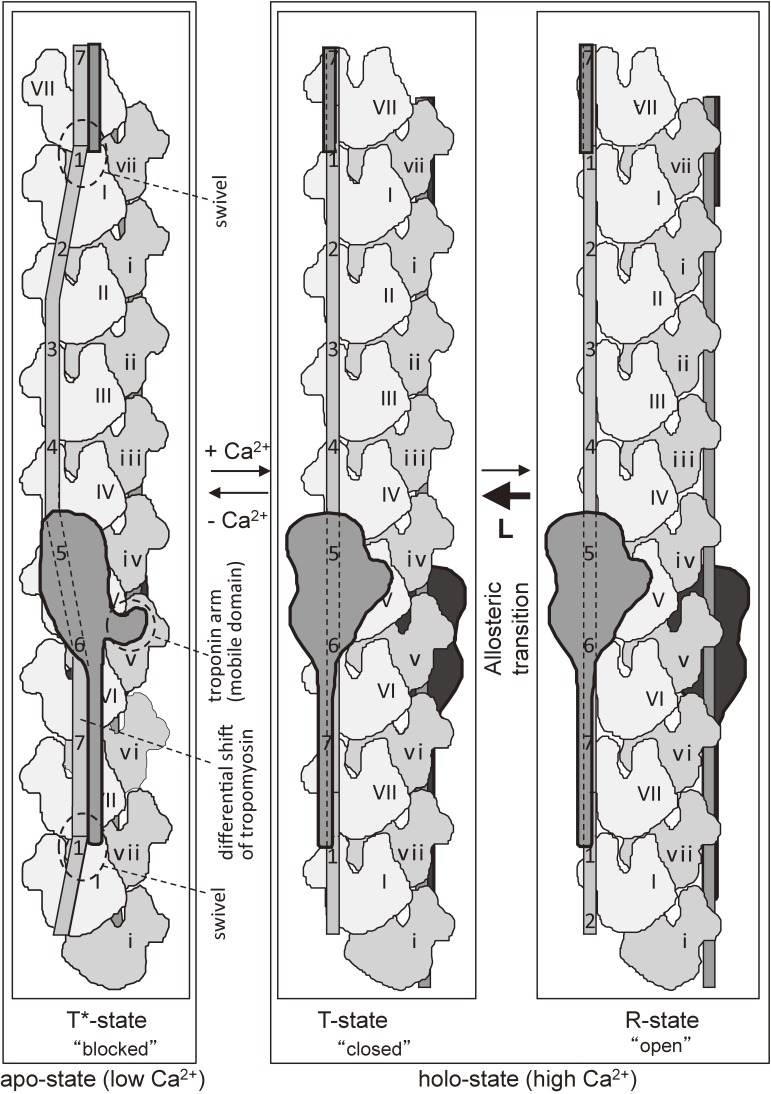
Proposed structural three-state model for Ca2+-regulation.84) To simplify the illustrations, actin helix was untwisted so that two strands become parallel. At low Ca2+ (“blocked” T* state), the swivel119) formed by tropomyosin junction and troponin-T compensate for the differential shift84) of tropomyosin induced by the tight association of the troponin arm84) with actin. The differential shift can explain why the inhibition at low Ca2+ is not perfect. At high Ca2+ (holo-state), there are two states, a “closed” T-state and an “open” R-state as proposed on the basis of biochemical data.130,131) The structure at high Ca2+ shown in the right panel of Fig. 7A represents a “closed” T-state, whereas the position of tropomyosin in the actin-tropomyosin-myosin S1-ADP-Vi complex shown in Figs. 6A and 6D55) is located nearer to the groove of actin double helix and represents that in an “open” R-state, because the binding of myosin induces the “close”-to-“open” transition. This position is near to Ala-230 of actin, of which the mutation to tyrosine induced higher Ca2+-activation in the presence of tropomyosin and troponin, which is an important feature of the “open” R-state.132–134)