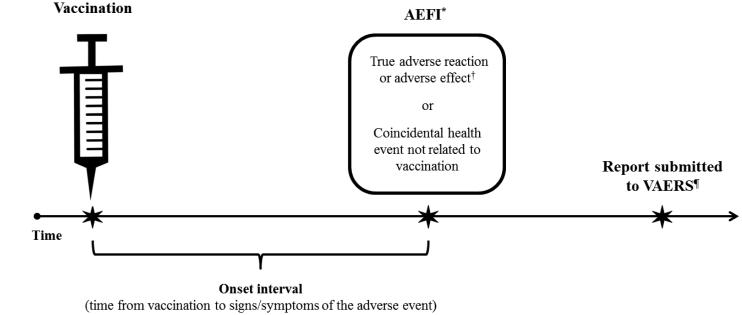
Figure 1. Adverse event following immunization (AEFI) and the VAERS reporting timeline.
*“Adverse event following immunization” (AEFI) indicates only that the event happened after vaccination (i.e., a temporal association).
†“Vaccine adverse reaction” and “vaccination adverse effect” are also AEFIs, but imply that the vaccine caused the event (i.e., a causal association).
¶There are no deadlines or time limits for the submission of a VAERS report, but reports should be submitted promptly after an adverse event occurs to facilitate surveillance and review. The National Vaccine Injury Compensation Program (VICP) is administered by the Health Resources and Services Administration (HRSA). The VICP is separate from the VAERS program and reporting an adverse event to VAERS does not constitute filing a claim for compensation to the VICP (see www.hrsa.gov/vaccinecompensation/index.html).