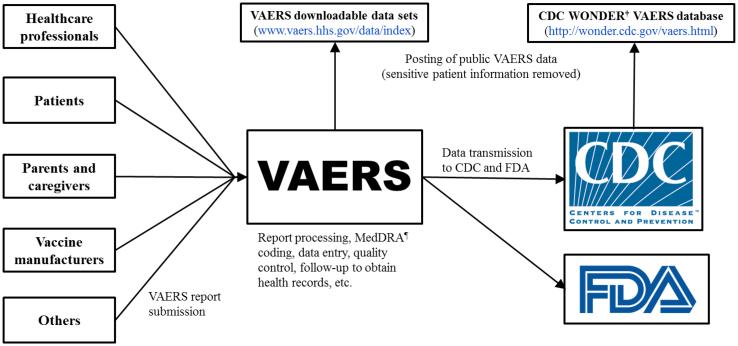
Figure 2. Vaccine Adverse Event Reporting System (VAERS) report submission* and data flow.
*During the time period 2011-2014, healthcare professionals submitted 38% of U.S. reports, patients and parents submitted 14%, vaccine manufacturers submitted 30%, and others (e.g., friends/acquaintances of the patient, 3rd party reporters who became aware of adverse events from the media, lawyers, etc.) submitted 12% (CDC unpublished data). There is variability in reporter type across different types and brands of vaccines.
†Wide-ranging Online Data for Epidemiologic Research
¶Medical Dictionary for Regulatory Activities