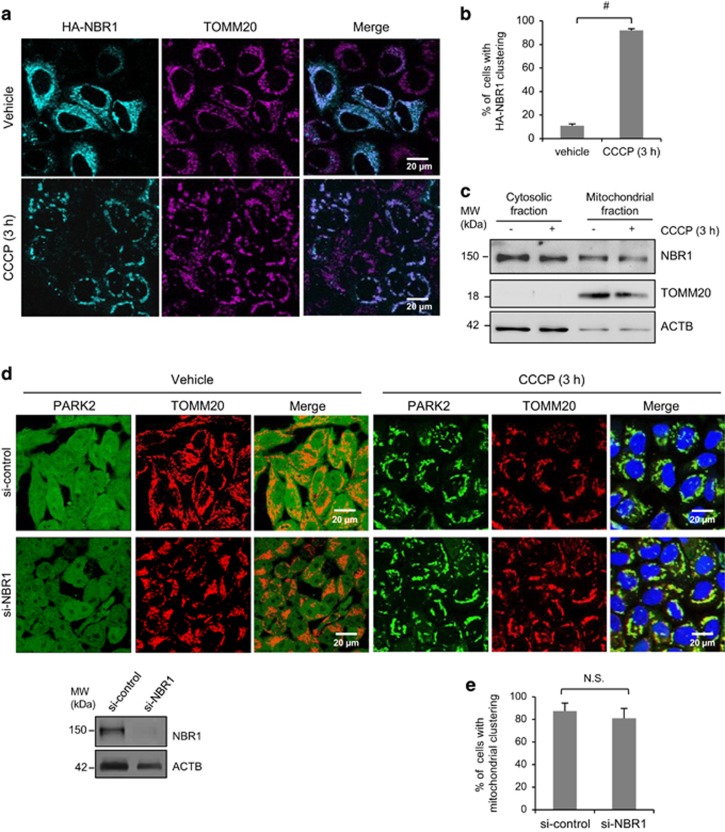
Figure 2.
NBR1 is not required for CCCP-induced, PARK2-dependent mitochondrial clustering. (a) HeLa cells stably expressing EGFP-Myc-PARK2 were transiently transfected with HA-NBR1 for 24 h, followed by vehicle or CCCP (10 μM) treatment for 3 h. Cells were double immunostained with anti-HA (cyan) and anti-TOMM20 (magenta) antibodies. (b) The percentage of cells with HA-NBR1 clustering following 3 h CCCP treatment over total HA-NBR1-positive cells in (a) is shown (at least 30 cells were counted for each image, mean±S.D., n=3 images). #P<0.01. (c) HeLa cells stably expressing EGFP-Myc-PARK2 were incubated with vehicle or CCCP (10 μM) for 3 h, followed by the isolation of cytosolic and mitochondrial fractions. Western blotting was performed to examine cellular distribution of NBR1 under basal and CCCP-stimulated conditions. TOMM20 and ACTB/β-actin were used as mitochondrial and cytosolic markers, respectively. The images are representative of two independent experiments. (d) PARK2 stably expressing HeLa cells were transiently transfected with control siRNA (si-control) or siRNA against NBR1 (si-NBR1) for 48 h, followed by vehicle or CCCP (10 μM) treatment for 3 h. Cells were immunostained with anti-TOMM20 antibody (red). Nuclei were counterstained with DAPI (blue). The si-NBR1 knockdown efficiency was assessed by western blotting using anti-NBR1 antibody. ACTB/β-actin was probed as a loading control. (e) Immunostaining results in (d) were quantified as percentages of cells with mitochondrial clustering over total number of cells (at least 30 cells were counted for each image). Results are presented as mean±S.D., n=3 images. NS, not significant