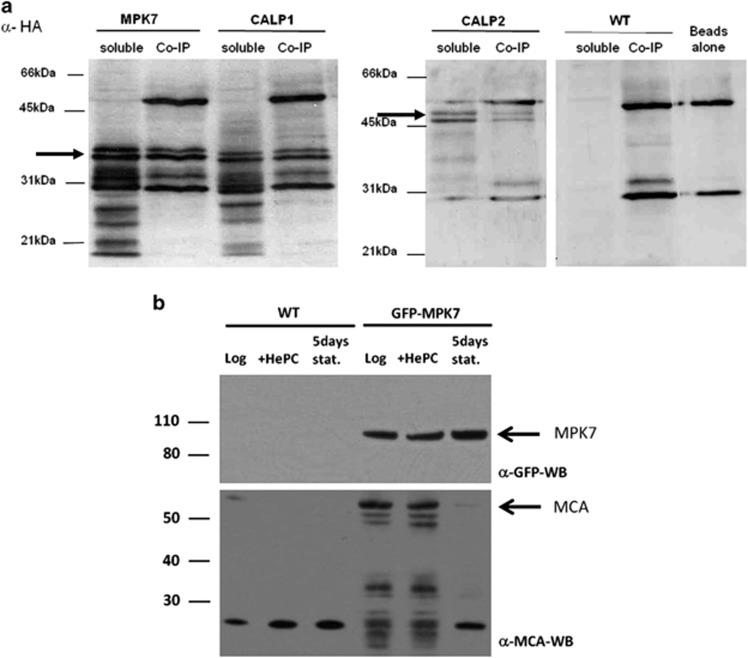
Figure 6.
Confirmation of the interaction of MCA with LmaMPK7 and CALP. (a) Co-immunoprecipitation, confirming the interaction of LmaMPK7 and CALP with LmjMCA. Western blot of lysates (soluble) and immunoprecipitated beads (Co-IP), revealed with an anti-HA antibody, after cell lysis and incubation with protein-sepharose beads. The two prominent bands at around 50 and 30 kDa represent the heavy and light chain of the myc-antibody, respectively that has been coupled to the sepharose beads (beads alone). Out of five tested proteins, only two could be co-immunoprecipitated at a size of about 37 and 47 kDa (black arrows), namely the interacting sequence of LmaMPK7 and a first sequence of CALP (CALP1) at 37 kDa and a second sequence of CALP (CALP2) at 47 kDa. An unspecific band appears at around 32 kDa (WT). Two bands appeared for all of the co-immunoprecipitated proteins on which shrimp alkaline phosphatase treatment or incubation of the membrane with an ubiquitin antibody had no effect. The probable cleavage products obtained for the soluble fractions of LmaMPK7 and CALP1 could be reduced by adding a protease inhibitor cocktail, leupeptin, that inhibits LmjMCA64 and pepstatin as shown for CALP2, although much less proteins were present. Two bands were nevertheless appearing for the co-immunoprecipitated proteins. (b) Pull-down assay, confirming the interaction of LmaMPK7 with LmjMCA. Total proteins were extracted from non-transfected L. major WT cells and WT parasites expressing GFP-tagged L. major MPK7 (GFP-MPK7) obtained from logarithmic cultures that were untreated (log) or treated for 4 h with 40 μM of miltefosine (+HePC), or from day 5 stationary phase (stat). GFP-MPK7 was purified, separated by SDS-PAGE and analyzed by immunoblotting with anti-GFP (α-GFP-WB) and anti-MCA antibodies (α-MCA-WB). Molecular mass standards are indicated in kilodaltons (kDa) (GFP-MPK7: 94 kDa, MCA: 54 kDa). The <30 kDa band represents a nonspecific signal as it is revealed in the control pull-down using anti-GFP antibody in combination with lysates from untransfected WT parasites