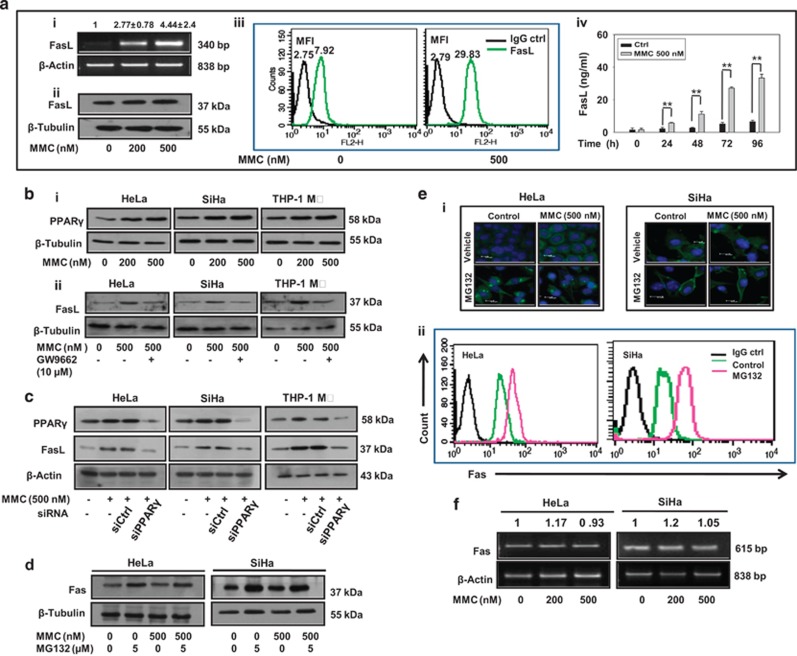
Figure 2.
MMC induces death ligand expression via PPARγ and proteasomal inhibition increases level of death receptors in cervical cancer cells. (a) Analysis of expression of FasL in MMC-treated THP-1 MΦ. (i) Semi-quantitative RT-PCR for FasL mRNA. THP-1 MΦ were treated with indicated concentrations of MMC, and processed for RT-PCR. β-Actin was used as a loading control. Data are mean±S.D., and are representative of three independent experiments. (ii) THP-1 MΦ were treated with indicated concentrations of MMC, and whole-cell lysate were subjected to western blotting for FasL. (iii) Flow cytometric analysis of FasL expression. THP-1 MΦ were treated with MMC as described above. Untreated or MMC-treated cells were probed with FasL primary antibody or IgG control (1 : 100), and further with PE-conjugated secondary antibody (1 : 200). Cells were then washed with PBS, and FasL expression was analyzed by flow cytometry. (iv) Sandwich ELISA for quantification of sFasL from untreated and MMC-treated THP-1 MΦ at indicated time points. (b) Analysis of involvement of PPARγ in the regulation of FasL expression. (i) Western blot analysis of PPARγ in MMC-treated cells. HeLa, SiHa and THP-1 MΦ were plated in 35 mm culture dishes. After 24 h, MMC treatment was given at indicated concentrations, and cells were further incubated for 24 h. Cell lysates were then subjected to SDS-PAGE and western blotting for PPARγ. (ii) Effect of PPARγ inhibition on FasL expression. HeLa, SiHa and THP-1 MΦ were plated in 35 mm culture dishes. After 24 h, cells were pretreated with GW9662 (10 μM) for 2 h. Thereafter, MMC (500 nM) treatment was given and cells were incubated for 22 h. Whole-cell lysates were subjected to western blotting for FasL. (c) Effect of knockdown of PPARγ on MMC-induced expression of FasL. HeLa, SiHa and THP-1 MΦ were transfected with control siRNA or PPARγ siRNA for 15 h, and allowed to grow for a further 15 h. Control siRNA and PPARγ siRNA-transfected cells were exposed to MMC for 24 h, and cells were collected for western blot analysis of PPARγ and FasL. (d) MG132-induced expression of Fas. HeLa and SiHa cells treated with MMC and/or MG132 for 24 h. Western blot analysis of whole-cell lysates subjected to SDS-PAGE and probed for Fas. (e) Analysis of expression and localization of Fas in MG132-treated cervical cancer cells. (i) Immunofluorescence staining of HeLa and SiHa cells. Cells were treated with MMC and/or MG132 for 24 h, washed twice, then fixed and permeabilized with 4% paraformaldehyde and 1% Triton X-100 respectively, and blocked with 5% FBS. Cells were further incubated with anti-Fas primary antibodies (1 : 100) for 2 h and subsequently stained with FITC-conjugated secondary antibodies (1 : 200) for 1 h. (ii) Flow cytometric analysis of Fas expression in cervical cancer cells. HeLa and SiHa cells were treated with MG132 for 24 h. Untreated or MG132-treated cells were probed with primary antibody against Fas or IgG control (1 : 100) for 1 h, and further with PE-conjugated secondary antibody (1 : 200) for 30 min. Cells were then washed with PBS, and Fas expression was analyzed by flow cytometry. (f) Semi-quantitative RT-PCR for Fas mRNA in MMC-treated cervical cancer cells. HeLa and SiHa cells were treated with indicated concentrations of MMC for 24 h, and were processed for RT-PCR. β-Actin was used as a loading control