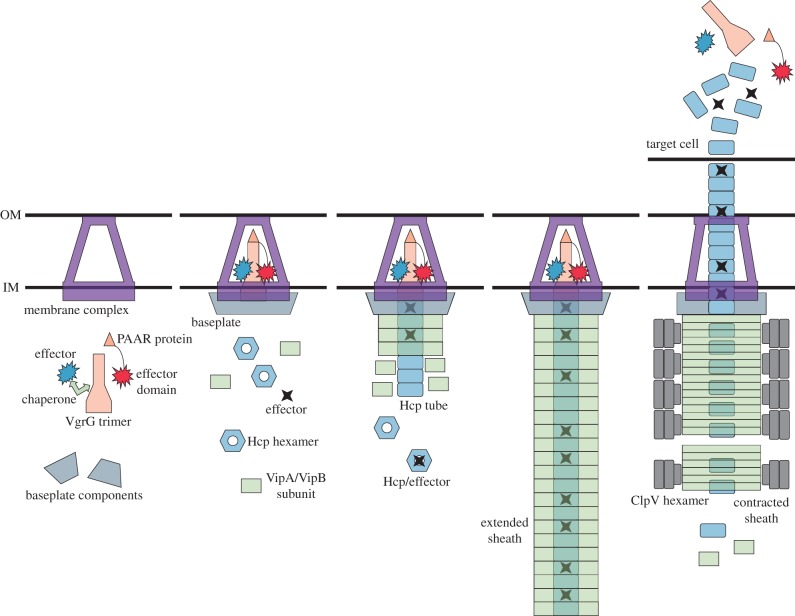
Figure 1.
T6SS mode of action. Assembly of T6SS in an extended ‘ready to fire’ conformation starts by the assembly of a membrane complex composed of TssJLM. Effector domains can be either present on VgrG C-terminus and PAAR C- and N-termini or be preloaded onto VgrG/PAAR spike complex, optionally with an assistance of non-secreted chaperones. VgrG/PAAR/effector complex possibly together with TssEFG and K proteins form the baseplate in a conformation that initiates assembly of tube and sheath. Hexameric rings of Hcp, potentially with bound effectors, assemble to a long rigid tube that serves as a template for the assembly of an extended VipA/VipB sheath. Potential conformational change in the baseplate/membrane complex triggers the sheath contraction, which pushes the Hcp tube with the VgrG/PAAR spike and associated effectors from the cell to an extracellular space or across a target cell membrane. The contracted sheath is specifically recognized by ClpV ATPase, which unfolds the subunits and thus recycles them for a new round of assembly of an extended sheath.