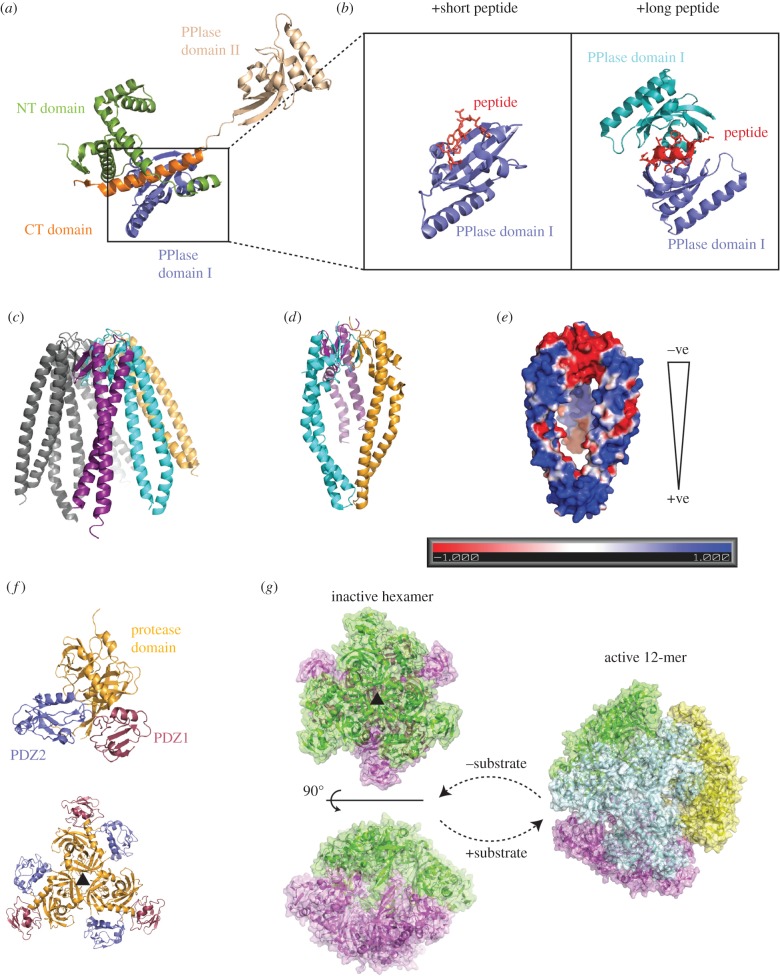
Figure 2.
Structures of the major periplasmic chaperones involved in OMP biogenesis. (a) The overall structure of full-length SurA alone and in (b), the PPIase domain bound to two different length peptides. (c) The structure of prefoldin with three of the six monomers coloured. The structure of Skp with each monomer coloured as for prefoldin (d) and the electrostatic surface potential calculated by ABPS (e). (f) The trimeric building block of DegP binds to another trimer to form an inactive hexamer (g, left) in the absence of substrate. Upon substrate addition, the trimers rearrange and form a larger cage-like structures, such as a 12-mer (g, right).