Figure 2.
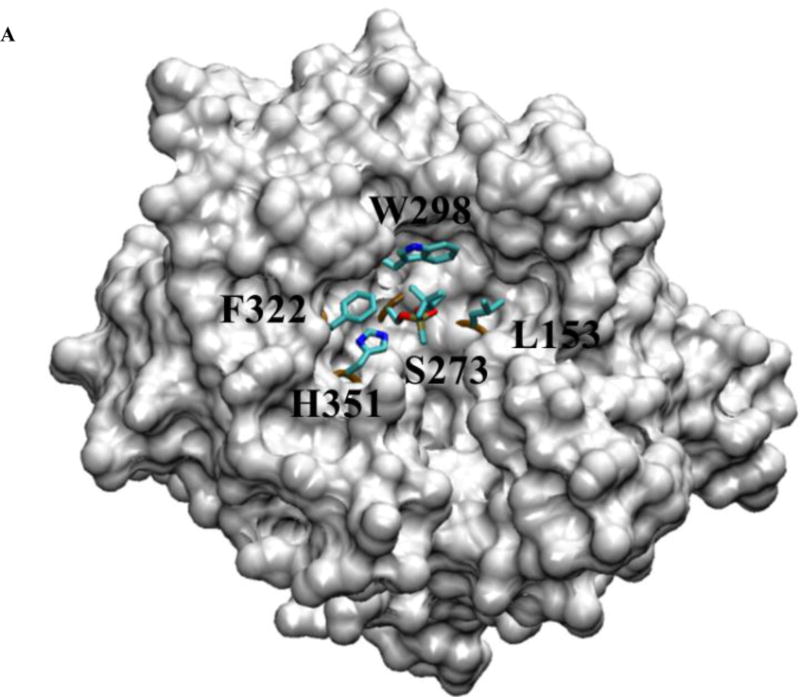
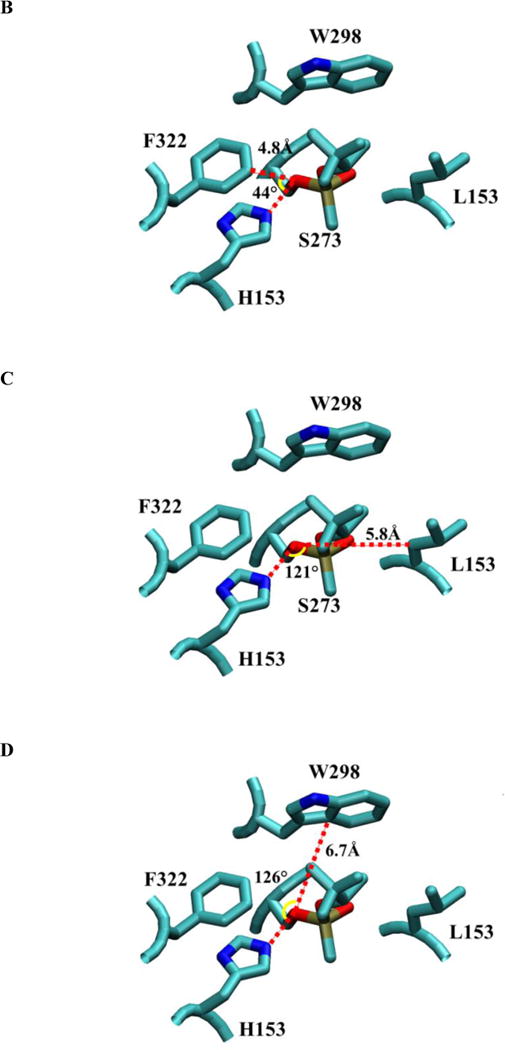
The structure of pPAF-AH complexed with soman (9). Panel A shows a space-filled, partially cut-down structure of pPAF-AH with soman adducted to S273 and shows the residues surrounding the active site S273 and H351. F322, W298 and L153 are sites for mutagenesis. Panel B shows the approximate angle (yellow arc) and distance (red line) to the S273 Oγ for F322 if we assume S273 is the vertex and the direction to the active site histidine (H351) forms one side (red line). F322 is closest to S273 Oγ at 4.8 Å and sits close to H351 at approximately 44°. Panel C shows L153 is offset from H351 by approximately 121°, but it is more than 5.8 Å away from S273 Oγ. Panel D shows W298 offset from H351 by approximately 126°, and at a position of 6.7 Å from S273 Oγ.
