Fig. 1. Treatment schema and IL13-zetakine+ CTL manufacturing.
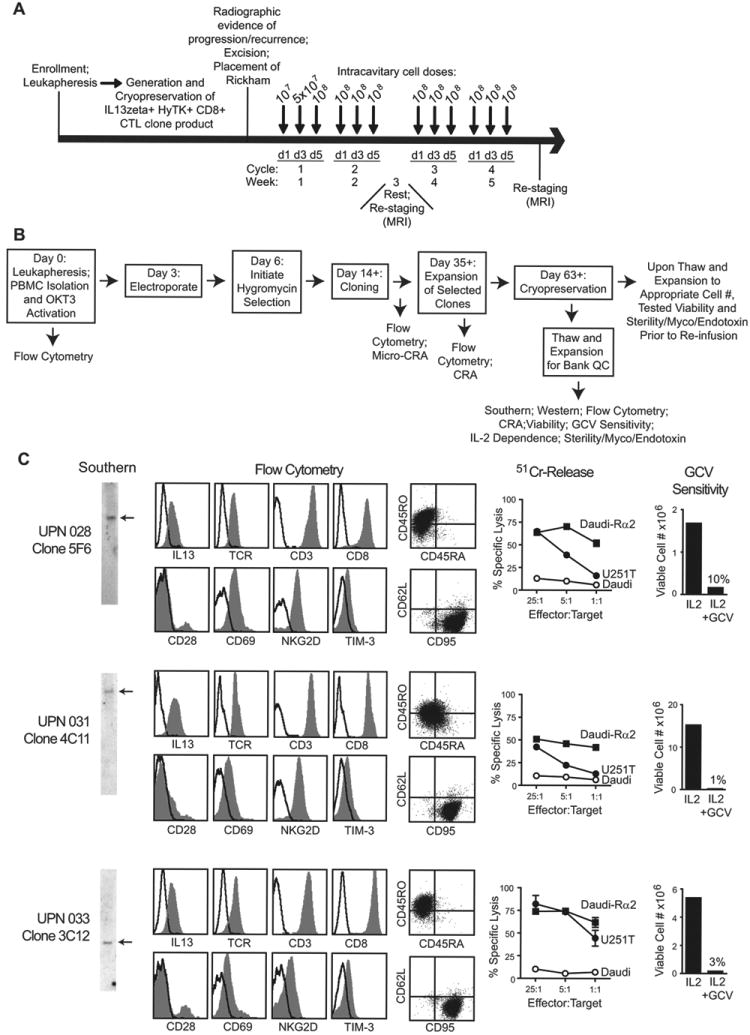
A, Four weekly cycles of intracavitary cell doses were administered after enrolled patients experienced recurrence and underwent tumor excision with placement of a Rickham catheter. Patients had a week of rest for brain imaging between cycles 2 and 3. B, Schematic of the manufacturing process, with day of each step(s) and in-process analyses indicated. CRA, chromium release assay; GCV, ganciclovir; Myco, mycoplasma; OKT3, a CD3 agonistic antibody used to activate T cells; PBMC, peripheral blood mononuclear cell. C, Characterization of the three cell products administered to patients with recurrent glioblastoma. Depicted from left to right: Southern blots of T cell genomic DNA using a hygomycin-specific probe (detecting HyTK selection/suicide fusion gene) showing existence of single bands as indicated by arrows; flow cytometric analysis of surface CAR expression using anti-IL13 antibody, or the T cell markers TCR-αβ, CD3, CD8, CD28, CD45RO, CD45RA, CD62L, CD69, CD95, NKG2D, and TIM-3 where isotype control staining is indicated with the open histograms or quadrant placement; ability of CTL clones to lyse IL13Rα2+ targets U251T and Daudi-13Rα2, but not non-engineered Daudi cells, determined in a 4-hour 51Cr-release assay; and ganciclovir (GCV) sensitivity using a flow-cytometry based assay for viable cell numbers after 14 days of culture with either rHuIL-2 or rHuIL-2 + GCV.
