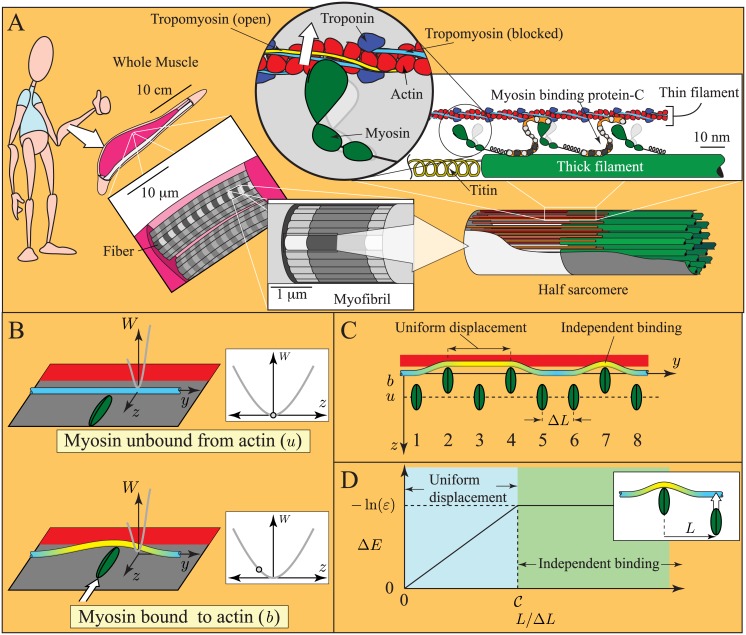
Fig 1. Cartoons of muscle and the model of local coupling.
A. Size scales in muscle. Muscle contracts due to the formation of transient links between thick filaments (made primarily of the protein myosin) and thin filaments (made of the proteins actin, troponin and tropomyosin). Contraction is regulated by troponin and tropomyosin. After binding calcium, troponin moves tropomyosin from a position where myosin binding to actin is obstructed (blue, blocked position) toward a position where myosin binding is unhindered (yellow, open position). B. Assumptions of the continuous flexible chain model [15–17]. Troponin-tropomyosin (Tn-Tm) is a slender, infinite, linearly elastic beam constrained to a plane and in a potential well, W(z). Myosin binding to actin induces a displacement of the Tn-Tm beam into the open position (yellow), and locally deforms it. C. Simplifying assumptions of the model. When nearby myosin molecules bind to actin (molecules 2 and 4), they uniformly displace the intervening Tn-Tm beam into the open position. When distant myosin molecules bind to actin (molecules 4 and 7), they each induce independent deformations of the Tn-Tm beam. D. Energy change of the thin filament (ΔE) due to myosin binding, as a function of the distance to a bound myosin (L). When L is small, Tn-Tm is displaced uniformly, and ΔE increases linearly with L (blue region); when L is large, each myosin displaces Tn-Tm independently, and ΔE is independent of L (green region). If the transition between these regimes is abrupt, the curve is defined by two non-dimensional parameters, and ε. Note: in the presence of calcium, the Tn-Tm beam moves into the closed state (not pictured), which changes ε, but not .