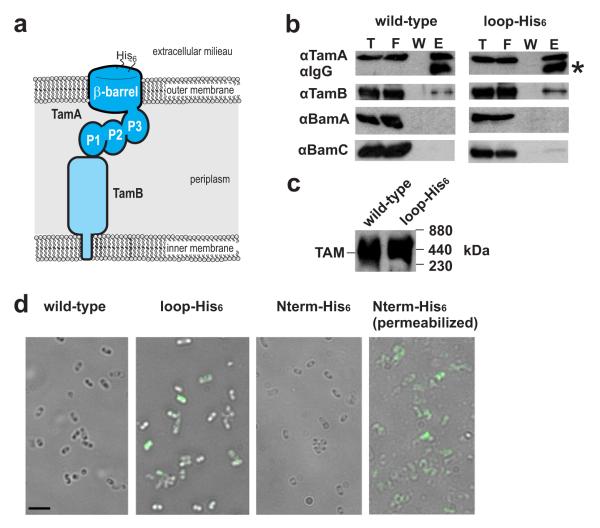
Figure 1. Insertion of a His6 sequence into an external loop of TamA.
(a) Topology of TamA: a β-barrel domain embedded in the outer membrane and three POTRA domains (P1, P2 and P3) exposed to the periplasm of the bacterial cell. Orientation of the nickel-binding sequence (His6) is shown. (b) Membrane extracts prepared from E. coli expressing TamA (wild-type) or TamA with “loop-His6” sequence (Supplementary Fig. 1), were subject to immunoprecipitation with the anti-TamA and ProteinA-Sepharose, followed by detection with the indicated antibodies after SDS-PAGE. T,10% of the total proteins; F, 10% of the flow-through fraction; W, 20% of the last three wash fraction; E, 100% of the eluted proteins. (c) Alternatively, to assess interaction of TamA and TamB subunits as the ~440 kDa TAM, membrane extracts prepared from E. coli expressing either TamA or the loop-His6 form of TamA, were analyzed by BN-PAGE and immunoblotting. (d) E. coli expressing TamA (wild-type) without a His6 sequence, TamA with the loop-His6 epitope, or the control N-terminal N-His6-epitope (as documented in Supplementary Fig. 1) were subject to immunofluorescence microscopy. A second sample of cells was permeabilized to provide access to the periplasm for immunostaining. Scale bar, 10 μm.