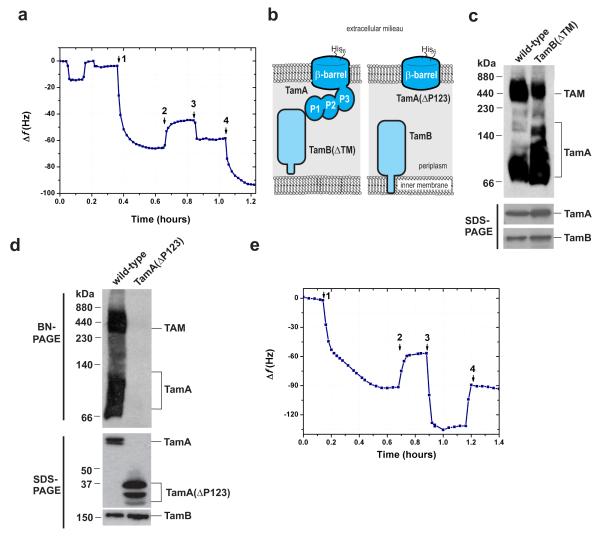
Figure 2. A synthetic membrane incorporating the outer membrane protein TamA.
(a) QCM-D measurements show the frequency response, indicative of mass changes, to [1] the attachment of TamA to the gold-surface, [2] washing away of detergent with a positive frequency shift, [3] reconstitution of the membrane layer with phospholipids, followed by rinsing at t=0.95 hrs and [4] the addition of purified TamB revealing a frequency response of ~30Hz. (b) Schematic summary of TamA and TamB constructs used in QCM(D) analysis. (c) Membrane extracts from E. coli expressing the tamAB operon, or a modified tamAB operon which expresses TamBΔTM, were analysed by BN-PAGE followed by immunoblotting for TamA. Samples of each extract were assessed in parallel by SDS-PAGE (lower panel) to determine the relative level of TamA and TamB/TamBΔTM in each extract. (d) Total membranes from E. coli expressing the tamAB operon (containing wild-type TamA-His6) or a modified tamAB operon (containing TamAΔP123-His6) were analysed by BN-PAGE and immunoblotting (upper panel) to assess the formation of the TAM, and SDS-PAGE and immunoblotting (bottom panel). to determine the relative level of TamA/ TamAΔP123 and TamB in each extract. (e) QCM-D measurements show the frequency response, indicative of mass changes, for membranes reconstituted from TamA(ΔP123). [1] detergent solubilised TamA(ΔP123) was added to the gold surface [2] washing of detergent with positive frequency change and [3] reconstitution of the membrane layer after addition of POPC, followed by rinsing away unbound POPC at t=1 hr revealing a positive frequency change and [4] the addition of TamB indicating no binding to TamA(ΔP123).