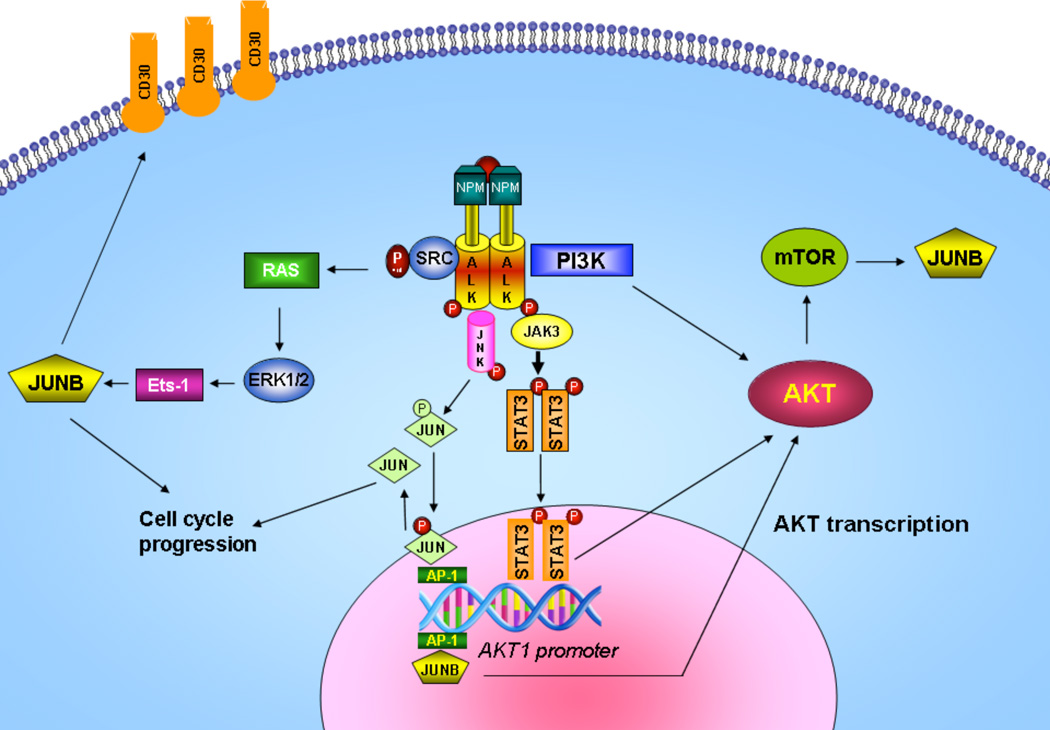
Figure 7. Proposed model of AP-1/STAT3/AKT crosstalk mechanism in ALCL.
On the basis of our findings shown here and the current knowledge from published data, we propose the following model in ALK+ ALCL. The PI3K/AKT pathway is constitutively active in ALK+ ALCL due largely to the direct physical interaction of NPM-ALK with PI3K. This PI3K-dependent pathway is highly conducive to determining the phenotype of ALCL, which is characterized by apoptosis and cell cycle deregulation. In addition, AKT is regulated at the transcriptional level by AP-1 and STAT3 through protein-DNA interactions in the upstream or downstream region of the AKT1 promoter, respectively. AP-1 members of the Jun family, JUNB and CJUN, play important roles in the pathogenesis of ALK+ ALCL. CJUN is activated through phosphorylation by JNKs in a NPM-ALK-dependent manner, whereas JUNB levels are regulated by NPM-ALK at the transcriptional level through MEK/ERK and Ets-1 transcription factor, and at the translational level through mTOR15, 16. Moreover, the JAK/STAT pathway is directly activated by NPM-ALK, and STAT3 is overexpressed and highly activated in ALK+ALCL22, 50. JUNB/CJUN and STAT3, in turn transcriptionally regulate AKT1 and possibly AKT2 and AKT3, thus contributing to AKT overexpression in ALK+ ALCL. Taken together, in addition to the ALK kinase-associated activation of downstream kinases, a functional crosstalk between AP1 transcription factors, JAK/STAT and AKT exists and further contributes to cell cycle and apoptosis deregulation and ultimately to oncogenesis of ALK+ ALCL.