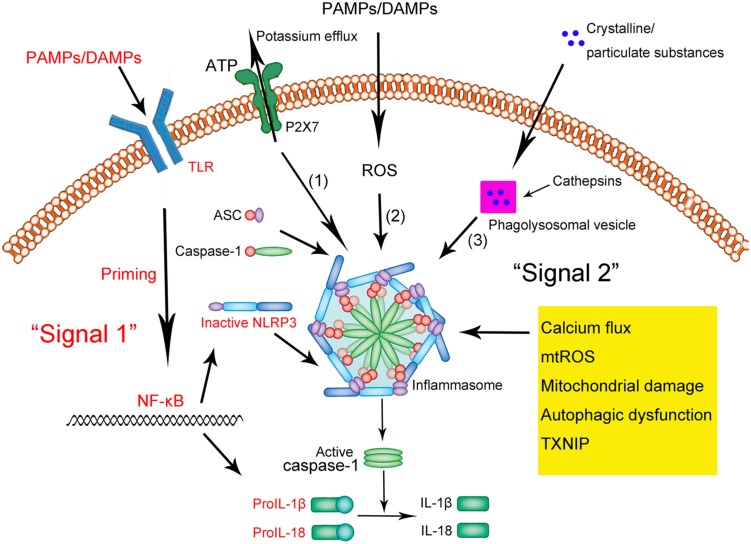
FIGURE 1.
Schematic illustration of the NLRP3 inflammasome activation. Upon exposure to pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs), Toll-like receptors (TLRs) are phosphorylated and subsequently activate NF-κB. In the nucleus, NF-κB promotes the transcription of NLRP3, proIL-1β, and proIL-18, which, after translation, remain in the cytoplasm in inactive forms. Thus, this signal (depicted in red as “Signal 1”) is a priming event. A subsequent stimulus (shown as “Signal 2” in black) activates the NLRP3 inflammasome by facilitating the oligomerization of inactive NLRP3, apoptosis-associated speck-like protein (ASC), and procaspase-1. This complex, in turn, catalyzes the conversion of procaspase-1 to caspase-1, which contributes to the production and secretion of the mature IL-1β and IL-18. Three models have been proposed to describe the second step of inflammasome activation: (1) Extracellular ATP can induce K+/potassium efflux through a purogenic P2X7-dependent pore, which, leads to the assembly and activation of the NLRP3 inflammasome. Calcium flux is also involved in this process. (2) PAMPs and DAMPs trigger the generation of ROS that promote the assembly and activation of the NLRP3 inflammasome. (3) Phagocytosed environmental irritants form intracellular crystalline or particulate structures leading to lysosomal rupture (magenta box) and release of lysosomal contents like cathepsin B. These induce NLRP3 inflammasome assembly and activation. In addition, other factors and mechanisms have been implicated in the assembly and activation of the NLRP3 inflammasome, including mitochondrial damage, autophagic dysfunction, and thioredoxin-interacting protein (TXNIP).