Abstract
Even when hunting in groups is mutually beneficial, it is unclear how communal hunts are initiated. If it is costly to be the only hunter, individuals should be reluctant to hunt unless others already are. We used 70 years of data from three communities to examine how male chimpanzees ‘solve’ this apparent collective action problem. The ‘impact hunter’ hypothesis proposes that group hunts are sometimes catalysed by certain individuals that hunt more readily than others. In two communities (Kasekela and Kanyawara), we identified a total of five males that exhibited high hunt participation rates for their age, and whose presence at an encounter with red colobus monkeys increased group hunting probability. Critically, these impact hunters were observed to hunt first more often than expected by chance. We argue that by hunting first, these males dilute prey defences and create opportunities for previously reluctant participants. This by-product mutualism can explain variation in group hunting rates within and between social groups. Hunting rates declined after the death of impact hunter FG in Kasekela and after impact hunter MS stopped hunting frequently in Kanyawara. There were no impact hunters in the third, smaller community (Mitumba), where, unlike the others, hunting probability increased with the number of females present at an encounter with prey.
Keywords: cooperation, chimpanzee, hunting, collective action, predation, by-product mutualism
1. Introduction
Understanding the causes and consequences of cooperation has long been a central research goal of economists [1], behavioural ecologists [2] and anthropologists [3,4]. We use ‘cooperation’ to describe cases in which at least two individuals act together in a way that increases their individual fitness relative to acting alone (joint action for mutual benefit [5,6]). Cooperative hunting occurs in several taxa, including cetaceans [7,8], social carnivores [9–12], non-human primates [13,14], birds [15] and humans [16]. The immediate benefits of cooperative hunting come in many forms. During periods when prey is scarce, large groups of African lions (Panthera leo) achieve greater per capita meat intake than small groups do [17]. In African wild dogs (Lycaon pictus), chase distances decrease as group sizes increase [10], resulting in more net energy per dog, even though smaller groups may actually acquire more kilograms of meat per hunt [18]. In cases when hunting in groups comes at a net caloric cost, a hunter may receive a benefit in a different ‘currency’, including rare micronutrients [19–21], or social favours such as grooming or coalitionary support ([22] but see [23,24]).
Despite considerable research on the benefits of hunting in groups, few studies have explicitly addressed how such hunts are initiated. This is an important oversight, because even if communal hunting is ultimately beneficial to each participant, receipt of this pay-off is contingent on the behaviour of others. If the costs of being the only hunter are sufficiently high and participation by others is uncertain, then individuals should be reluctant to initiate a hunt. Thus, hunting in groups appears vulnerable to a collective action problem stemming from the fact that the costs are incurred by a subset of the group while the benefits are distributed more widely [1,25]. This situation presents an opportunity for individuals to take advantage of others, either by not participating at all (‘strong free-riding’) or contributing less than their share (‘weak free-riding’) [26–28]. In the case of cooperative hunting, the costs come from expending energy and encountering danger (from being attacked, or from falling) while chasing and confronting prey. Why initiate a hunt when others could do so instead?
We examine this question in chimpanzees (Pan troglodytes), which regularly engage in group hunts of red colobus monkeys (Procolobus spp.) wherever the two species are found together [14]. Chimpanzees live in large social groups (communities) that average 46.2 individuals (calculated from reference [29]), and exhibit high fission–fusion dynamics [30–33], whereby community members are found in subgroups (‘parties’, hereafter) that frequently change in size and composition. Red colobus monkeys are medium-sized (approx. 7–12 kg [34]), arboreal primates that live in groups averaging 36 individuals (range 9–82, calculated from reference [34], appendix 3.2, using data from four major long-term chimpanzee study sites (Gombe National Park, Tanzania; Kanyawara (Kibale National Park, Uganda); Ngogo (Kibale); Taï Forest, Côte d'Ivoire). At Ngogo [35] and Taï [36], chimpanzees may actively search for red colobus monkeys, while elsewhere encounters appear to occur by chance during routine activities (e.g. Kasekela (Gombe) [37] and Kanyawara; R. W. Wrangham, personal observations, 1987–2014).
At all sites, upon encountering a troop of red colobus monkeys (interchanged with ‘colobus’, hereafter), the probability of a hunt occurring (and succeeding) increases with male chimpanzee party size [13,14]. When a hunt occurs, many individuals (usually adult males) typically participate. Male colobus often cooperate to mob chimpanzee hunters [33,36], sometimes driving them to the ground [33]. Group hunts at East African sites (e.g. Kasekela, Kanyawara, Ngogo and Mahale Mountains National Park, Tanzania) are best described as simultaneous, individual efforts [21,38–41]. In one West African site (Taï), by contrast, Boesch & Boesch [36,38,42,43] have reported that hunts are often highly collaborative. According to their collaboration hypothesis, chimpanzees adopt specific, differentiated roles during a hunt. ‘Drivers’ chase colobus prey past ‘blockers’ (that position themselves at critical escape routes without actively trying to capture prey), towards ‘ambushers’ and ‘chasers’ that ultimately make the kill. These descriptions imply that in order to maximize the probability that the group succeeds, some hunters behave in a way that reduces their own chances of capturing a monkey himself (a true ‘team task’ [44]). A complex system of sharing reportedly ensures that those that make this immediate sacrifice are compensated for their efforts [36,38,42]. Such a system presumably requires advanced cognitive skills, including ‘social knowledge of what other hunters see and are able to do, as well as knowledge of the specific way they are going to react to this knowledge’ [42, p. 42].
However, Gilby & Connor [45] argue that a simple by-product mutualism (in which an individual's selfish actions incidentally benefit others [46–48]) can explain group hunting dynamics at both East and West African chimpanzee sites, including Taï. In this conceptual model, each hunter seeks to catch a monkey, rather than acting to increase the probability that the group as a whole succeeds [44]. As more individuals hunt, prey defences become increasingly diluted, thus reducing hunting costs for every hunter. Also, as female and juvenile monkeys flee, there are more opportunities to make a kill in the ensuing chaos. This shift in the perceived costs and benefits of hunting should prompt initially reluctant chimpanzees to hunt. As chimpanzees react to the actions of prey (and predator), what looks like a complex, coordinated division of labour may emerge [48]. Until it can be shown at Taï that a ‘blocker’ is not simply placing himself in a position where he is likely to capture a monkey that is fleeing from another hunter, we believe that the by-product mutualism framework cannot be rejected. Furthermore, the report that hunters at Taï frequently switch roles during hunts [42] is consistent with an ‘every chimpanzee for himself’ strategy. Hunters need only follow the simple rule, ‘hunt when others are hunting’, and via associative learning, develop an understanding that a fleeing monkey will change direction upon encountering another chimpanzee or a physical barrier. Such divisions of labour have been described among social predators such as African lions [49], African wild dogs [18], hyaenas (Crocuta crocuta) [50], grey wolves (Canis lupus) [51] and fish (interspecies cooperation between giant moray eels (Gymnothorax javanicus) and groupers (Plectropomus pessuliferus) [52]). Pending further tests of the collaboration hypothesis, therefore, we assume that group hunting of red colobus monkeys by chimpanzees can be explained by a by-product mutualism [21,45,53].
Accounting for group hunts as examples of by-product mutualism does not solve the initiation problem. However, previous research has indicated a possible solution, which is compatible with a by-product mutualism explanation. The ‘impact hunter’ hypothesis proposes that individuals vary in hunting motivation, and that a few males are willing to hunt by themselves [21,53]. While the source of this variation in hunting tendency is unknown, it promotes hunting by others. Specifically, ‘impact hunters’ attract the aggressive behaviour of adult male colobus aiming to deter predation. Once this happens, other chimpanzees find subsets of the colobus group that are relatively poorly defended, thereby taking advantage of more favourable odds that they themselves will make a kill. The impact hunter hypothesis has been supported by evidence that the presence of particular males at an encounter with colobus was positively associated with group hunting probability, even after controlling for male chimpanzee party size [21,53].
Theoretical support for this hypothesis comes from economic models of between-group competition that take into account individual variation in need, ability and participation costs [1,54]. Such heterogeneity should lead to ‘“exploitation” of the great by the small’ [1, p. 29]. Gavrilets [55] demonstrated that those who contribute the most towards production of collective goods (i.e. hunt initiators) are those (i) who are particularly skilled, or for whom (ii) the benefits are especially high or (iii) the costs relatively low. McAuliffe et al. [56] argue that the actions of such key individuals can explain puzzling cases of ‘positive matching’ in which individuals fail to reduce their contribution in response to increased cooperation by others.
Here, using several more years of data from two previously studied communities (Kanyawara, Kasekela) as well as 14 years of data from a third, smaller community (Mitumba, at Gombe), we determine whether the positive association between group hunting probability and the presence of certain individuals still holds. We then identify which of these individuals also exhibit high hunting rates for their age, and classify them as impact hunters (explained in detail below). Then we test the following predictions for the first time: (i) impact hunters will initiate hunts more often than expected by chance; (ii) when they hunt, impact hunters will be more likely than males of the same age to make a kill; and (iii) community-level hunting rates will decrease when an impact hunter is no longer alive or active.
2. Methods
(a). Research sites, data collection and extraction
(i). Kasekela and Mitumba (Gombe National Park, Tanzania)
Gombe National Park, located on the Eastern shore of Lake Tanganyika, is comprised of 35 km2 of evergreen riverine forest, woodland and grassland [57]. In 1960, Goodall [33] began to habituate the Kasekela chimpanzee community, which ranges in the centre of the park. Since the early 1970s (when the animals were fully accustomed to observers), field assistants have conducted almost-daily full-day focal follows [58] of adult chimpanzees [33,59], using checksheets to systematically collect several types of data, including party composition, female sexual swelling size (0, 1/4, 1/2, 3/4, 1) and all encounters with colobus monkeys (when observed within approximately 50 m of the focal chimpanzee, regardless of any interest in hunting). They write a continuous narrative of the behaviour of the focal chimpanzee, and of conspicuous group-level activities such as hunting [39,53,59]. When a hunt occurs, they record the identity of all chimpanzees observed to chase, and capture, monkeys. In 1994, using the same research protocol [60], field assistants began to follow chimpanzees in the smaller, neighbouring Mitumba community, which ranges in the northernmost regions of the park. The Mitumba chimpanzees were fully habituated to human observers by 2000.
The paper data from both communities are archived at the Jane Goodall Institute Research Center at Duke University. A relational database of information extracted from the checksheets and notes is maintained at Duke and at Arizona State University. The database also contains detailed demographic data. For every new infant, birthdate was estimated based on size and appearance. The earliest possible birthdate (BDMin [61]) was set as the last date the mother had been seen before giving birth. The latest possible birthdate (BDMax) was set as the first day the infant was seen. For all males born in Kasekela after 1962, the median difference between BDMax and BDMin was 7 days (n = 79, mean = 37, s.d. = 96.9). There were seven males that were born before the study began and were alive in 1976 (the start of this study; see below). Their ages were estimated based on morphological characteristics and familial relationships, and for only one (HM) did the range of possible birthdates span more than 2 years. The others were all juveniles when the study began. In Mitumba, for males born after 1994, the median difference between BDMax and BDMin was also 7 days (n = 13, mean = 12.6, s.d. = 15.1). There were five males born in Mitumba before 1994, with estimated ages ranging from 4 to 23 years old. For these males, the median difference between BDMax and BDMin was 730 days (mean = 1033.8, s.d. = 814.4).
Following Gilby et al. [39,53], we recorded the start times of all chimpanzee encounters with colobus monkeys in Kasekela (1976–July 2013) and Mitumba (2000–2014) from the checksheets and narrative notes. We determined the identity and number of all adult males (≥12 years old [39]), adult females (≥13 years old) and sexually receptive (‘swollen’) females (swelling state = 1) present at the beginning of each colobus encounter, ±15 min. From the narrative notes, we identified all hunt attempts as those cases in which at least one chimpanzee (male or female) climbed in active pursuit of a monkey. Following Gilby et al. [39,53], we excluded cases in which there was not enough information in the notes to determine whether or not a hunter climbed, as the descriptive term ‘hunt’ occasionally refers to running along the ground, intently watching the prey. We noted the identity of the first chimpanzee to hunt in cases where the description was sufficiently detailed and unambiguous. Finally, we recorded the identity of all hunters and for successful hunts (when at least one monkey was killed), those that captured prey.
(ii). Kanyawara (Kibale National Park, Uganda)
Kibale National Park, in Southwestern Uganda, covers an area of 766 km2 and consists of moist medium-altitude evergreen forest with areas of grassland, swamp and regenerating forest [62,63]. The Kanyawara chimpanzee community ranges in the Northwest region of the park. Data on the Kanyawara chimpanzees have been systematically collected since 1987 by trained Ugandan field assistants. When a party is located, they record sexual swellings (on a three-point scale from 1 to 3, with 3 being maximally tumescent) for all females. In addition, every 15 min, the field assistants record party composition, and since 1996, whether colobus can be detected within 100 m of the chimpanzees. Detailed data of all other behaviour, including hunting, are recorded as they occur. At Kanyawara, hunt attempts are defined as instances when a chimpanzee climbs to the height of the lowest monkey (similar to Boesch & Boesch [43]) and if one occurs, the field assistants spread out to ensure that they can observe the behaviour of as many individuals as possible. After a hunt, the field assistants, in consultation with each other and all other observers present, record information on an additional predation-specific checksheet.
The paper data from Kanyawara as well as a relational database of extracted information from the checksheets are maintained at Harvard University. From these records, we have identified all chimpanzee encounters with colobus (1996–August 2014) by querying the database for any 15 min scan when the chimpanzees were within 100 m of colobus that was not immediately preceded by another ‘positive’ colobus scan. We determined the number of adult males (≥12 years old), adult females (those who had been observed with a swelling of 3 on or before the observation date), and swollen females (swelling state = 3) present at the first 15 min scan of each encounter. We matched every observed hunt attempt to an encounter and then used the predation checksheets to identify which chimpanzees hunted, who hunted first and who was successful in capturing and killing prey.
The Kanyawara database contains detailed demographic data on all individuals who have been identified as members of the community since 1988. When a new individual is born in the community, the birthdate is estimated based on the size of the individual and the date the mother was last seen without the infant. Of the 22 adult males included in this study, 10 were born after 1988 and their birthdates were estimated to between 1 day and 1 year. For the 12 individuals born before 1988, birthdates were estimated between 1 and 5 years based on their appearance and body condition. G.I. Basuta, who studied the Kanyawara community from 1983 to 1985, provided helpful advice for estimating the ages of these older males.
(b). Analyses
All statistical analyses were performed using R v. 3.1.3 [64] using the lme4 [65] and multcomp [66] packages.
(i). Identifying impact hunters
We classified as impact hunters those males (i) whose presence at an encounter with red colobus monkeys was associated with increased group hunting probability and (ii) who participated in hunts more often than expected for their age.
To identify factors affecting hunting probability, we ran separate generalized linear models (GLMs) on the colobus encounter data from each site. For each encounter, the model estimated the probability of a hunt attempt (Y/N) using a binomial error structure and a logit link function. We included three main effects: (i) number of adult male chimpanzees present (‘male party size’), (ii) number of adult females present (‘female party size’) and (iii) the presence of at least one maximally swollen female (Y/N). Following precedents set by previous hunting studies [39,40,67], we treated each encounter as an independent event, because it is unlikely that the characteristics (i.e. party composition, location, etc.) of any two encounters would be similar enough to warrant incorporation of a random effect term. We considered a main effect to be statistically significant if p < 0.05.
We then ran a series of GLMs to assess whether the presence of certain males increased the probability of a hunt occurring. Following [53], we ran one GLM per adult male in each community, spanning the time when he was at least 12 years old until his death or the end of the study. As above, the response variable was hunt attempt (Y/N), and the model used a binomial error structure and logit link function. The main effects were those variables that were statistically significant in the above analysis (which differed by community), plus a single categorical predictor indicating that male's presence at particular encounter (Y/N). As each male experienced a unique set of encounters, we considered p-values less than 0.05 to be statistically significant, rather than apply a correction for multiple tests (following Gilby et al. [53]). We classified males whose presence was significantly positively associated with group hunting probability as potential impact hunters. Then, to build upon previous work [21,53], which relied solely on this correlation, we identified which of these potential impact hunters hunted more frequently than males of the same age. To do so, we needed to understand how hunting probability varied with age. For these analyses, we restricted our datasets to only those hunt attempts for which hunters were clearly identified. Given the fast-paced nature of these events, some hunters may have been missed because they were out of sight or hunted only briefly. However, there was unlikely to be any systematic bias in these omissions. We ran the following analyses separately for each study community. For each male present at a hunt attempt, we asked whether his age was associated with the probability that he participated in the hunt. We ran a generalized linear mixed model (GLMM) with hunt (Y/N) as the dependent variable, age (in 5 year blocks, starting at age 6) as a categorical main effect, and with chimpanzee ID and colobus encounter ID as random effects, using a binomial error structure and a logit link function. Then, we calculated the observed hunting probability (number of hunt participations/number of hunt attempts present for) of each potential impact hunter in each age class. We considered a chimpanzee to be more likely to hunt than the average male of the same age if his observed hunting probability was greater than the predicted value (+1 s.e. of the estimate) generated by the GLMM for a given age class.
(ii). Prediction 1: impact hunters will initiate hunts more often than expected by chance
At Kanyawara, observers are explicitly instructed to record the identity of the first chimpanzee to hunt, when possible. For each impact hunter, we calculated the proportion of group hunt attempts when he hunted first (provided that he hunted), grouping by the total number of hunters. We then used an exact paired Wilcoxon signed-ranks test to determine whether the actual values were greater than expected, using 1/X as the expected value, where X was the number of hunters.
At Kasekela and Mitumba, observers are not specifically asked to record which chimpanzee hunts first. However, we were often able to extract this information from the narrative notes. Therefore, when possible, we calculated the proportion of hunt attempts (with a known first hunter) when a potential impact male hunted first, provided that he participated.
(iii). Prediction 2: when they hunt, impact hunters will be more likely to make a kill than expected for their age
One of the findings of Gavrilets' model [55] was that those who contribute the most towards production of collective goods should be particularly skilled. Therefore, we ran another GLMM to ask whether impact hunters have unusually high success rates. For each male that was named as a hunter at a given hunt attempt, we asked whether he captured a monkey (Y/N), with age category as a fixed effect and male ID and colobus encounter ID as random effects, using a binomial error structure and a logit link function. As above, we compared the actual kill probability of impact hunters to the predicted probability and standard error generated by the model for each age category.
(iv). Prediction 3: community-level hunting rates will decrease after an impact hunter dies or stops hunting at above average rates
For each impact hunter that died during the study period, we compared overall group hunting rates (hunt attempts/colobus encounters) during the 4 years preceding his death with the 4 years following his death. For one impact hunter who no longer showed unusually high hunting rates after age 31, we compared group hunting probability in the 4 years before and after his 31st birthday (see §3e(i)). To account for possible changes in gregariousness (which can affect hunting rates), we calculated this value for each male party size, then used an exact Wilcoxon signed-ranks test to compare rates before and after the impact hunter's death or decline.
3. Results
A summary of colobus encounters, hunt attempts and successful hunts is provided in table 1. Encounters with colobus were more frequent at Kanyawara than at the other sites (3.73 per 100 h of observation versus 2.34 and 2.31 at Kasekela and Mitumba, respectively), perhaps owing to site-specific operational definitions of encounter (100 m at Kanyawara versus 50 m at Gombe). Nevertheless, the hunting rate (hunt attempts/encounters) at Kanyawara was much lower (7.9%) than at either Kasekela (64.7%) or Mitumba (48.0%). Success rate (successful hunts/hunt attempts) was higher at Kanyawara (61.3%) and Kasekela (62.3%) than at Mitumba (53.2%). The number of prey captured per successful hunt was higher at Kasekela (1.90) than at Kanyawara (1.28) or Mitumba (1.30).
Table 1.
Summary data from the three study communities. Data include all encounters with red colobus monkeys, regardless of chimpanzee party composition. For Kasekela and Mitumba, the numbers of red colobus encounters in parentheses represent those for which there was enough information to determine whether or not a hunt occurred (see text for further explanation). Hunting rates were calculated using these values.
| adult males |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| population | community | months of study | mean | range | IDs | red colobus encounters | encounters per 100 hr | hunt attempts (%) | successful hunts (%) | total prey (mean per succ. hunt) |
| Kibale | Kanyawara | 224 | 11.4 | 9–14 | 22 | 2461 | 3.73 | 194 (7.9) | 119 (61.3) | 152 (1.28) |
| Gombe | Kasekela | 451 | 10.4 | 6–14 | 35 | 2690 (2316) | 2.34 | 1498 (64.7) | 934 (62.3) | 1778 (1.90) |
| Mitumba | 163 | 2.9 | 2–5 | 7 | 613 (548) | 2.31 | 263 (48.0) | 140 (53.2) | 182 (1.30) | |
(a). Group hunting probability
In all three communities, the number of adult male chimpanzees present at a colobus encounter was significantly positively associated with hunting probability (table 2). At Kasekela, with all else equal (GLMM, see §2b(i)), the odds of hunting increased by 8% with each additional male, compared with much greater odds increases at Mitumba (72%) and Kanyawara (48%). The large effect at Mitumba is likely to be (at least partially) an artefact of the low number of males in this community. Indeed, when we re-ran the analyses for the other communities, using only encounters by parties with fewer than five males, the odds increases were greater (Kasekela: 28%, Kanyawara: 93%). At Mitumba, there was also a significant positive relationship between the number of adult females and hunting probability; all else equal, the odds of hunting increased 12% with each additional adult female (table 2). There was no effect of adult females on hunting at the other sites, even when we restricted the dataset to encounters by parties with fewer than five males (Kanyawara: p = 0.39; Kasekela: p = 0.17). At Kanyawara, there was a significant negative relationship between swollen female presence and hunting probability. If at least one swollen female was present, the odds of hunting were 22% lower than if no swollen females were present (table 2), all else equal. There was no association between the presence of swollen females and hunting in Mitumba or Kasekela.
Table 2.
GLMs of group hunting probability. In all three communities, there was a strong positive association between hunting probability and the number of adult males present at a red colobus encounter. At Mitumba, there was an additional positive effect of adult females on hunting likelihood. At Kanyawara, hunts were significantly less likely to occur if at least 1 swollen female was present. Bold italics indicate parameters that were statistically significant (p < 0.05).
| community | parameter | estimate | odds ratio | s.e. | Z | p-value |
|---|---|---|---|---|---|---|
| Kanyawara | males | 0.39 | 1.48 | 0.03 | 12.67 | <0.0001 |
| females | 0.03 | 1.03 | 0.03 | 0.93 | 0.35 | |
| swollen females (Y) | −0.72 | 0.49 | 0.19 | −3.7 | 0.0002 | |
| Kasekela | males | 0.08 | 1.08 | 0.02 | 4.64 | <0.0001 |
| females | 0.02 | 1.02 | 0.01 | 1.47 | 0.14 | |
| swollen females (Y) | −0.11 | 0.90 | 0.12 | −0.98 | 0.32 | |
| Mitumba | males | 0.54 | 1.72 | 0.1 | 5.5 | <0.0001 |
| females | 0.11 | 1.12 | 0.04 | 2.8 | 0.005 | |
| swollen females (Y) | −0.25 | 0.78 | 0.21 | −1.2 | 0.23 |
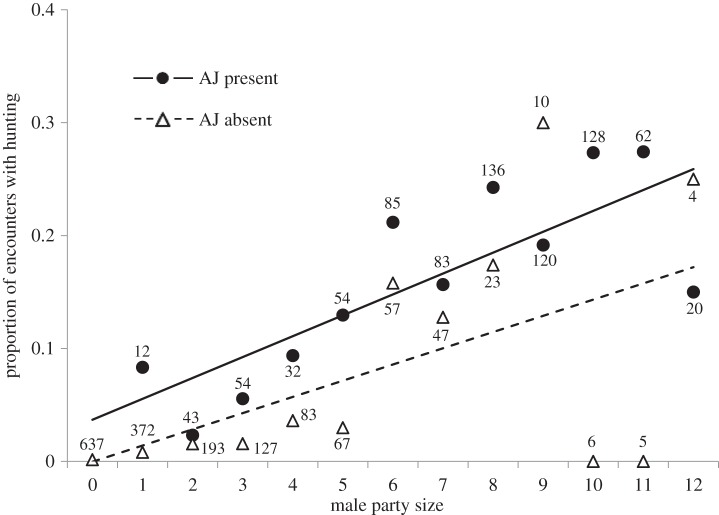
At Kanyawara, there were two adult males whose presence at a colobus encounter was strongly positively associated with the probability of a hunt occurring. Parties containing AJ hunted in 18.9% (157/830) of encounters, compared with only 2.3% (37/1594) when he was absent (figure 1 and table 3). This difference was statistically significant (GLM, controlling for adult male party size and the presence of at least one swollen female: odds ratio (OR) = 2.43, Z = 3.70, p = 0.0002, table 3). Similarly, parties containing adult male MS were more likely to hunt than parties without him (18.9% (135/713) versus 2.1% (26/1236), OR = 3.03, Z = 4.30, p = 0.00002, table 3). AJ and MS were both roughly the same age, and were present together in the community until MS's death in 2010. We considered AJ and MS as potential impact males, pending additional analyses, described below. None of the other 21 Kanyawara males who reached adulthood before or during the study period (1996–2014) was associated with increased hunting probability.
Figure 1.
The presence of impact hunter AJ and hunting probability, Kanyawara. Solid circles represent encounters with colobus at which AJ was present, versus open triangles, when he was absent. Numbers indicate number of encounters for each data point. Parties containing AJ were significantly more likely to hunt than those without him. Trend lines are for illustrative purposes only—data were analysed at the level of the encounter using a generalized linear model for binomial distributions (see text for details).
Table 3.
Summary of impact hunter analyses. For each chimpanzee listed, there was a significant, positive association between their presence at a colobus encounter and the probability that a hunt occurred. Bold italics type indicates those that consistently had above average hunting rates for their age and were therefore classified as impact hunters.
| community | chimp ID | sex | birth year | start year | end year | group hunt prob. when ID present | group hunt prob. when ID Absent | odds ratio | Z | p-value | hunt participation greater (1 s.e.) than mean for age? | hunted first more than expected |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kanyawara | AJ | M | 1974 | 1996 | 2014 | 0.19 (157/830) | 0.02 (37/1594) | 2.43 | 3.7 | 0.0002 | Y | Y |
| MS | M | 1975 | 1996 | 2010 | 0.19 (135/713) | 0.02 (26/1236) | 3.03 | 4.3 | 0.00002 | Ya | Y | |
| Kasekela | AO | M | 1979 | 1991 | 2013 | 0.70 (622/883) | 0.56 (379/679) | 1.34 | 2.1 | 0.04 | Yb | N |
| FG | M | 1953 | 1976 | 1982 | 0.75 (122/161) | 0.55 (62/113) | 3.13 | 3.0 | 0.003 | Y | data not available | |
| FR | M | 1976 | 1988 | 2013 | 0.69 (764/1108) | 0.56 (350/620) | 1.28 | 2.0 | 0.05 | Y | Y | |
| PX | M | 1977 | 1989 | 2013 | 0.70 (763/1093) | 0.54 (309/567) | 1.39 | 2.3 | 0.03 | N | N | |
| SL | M | 1983 | 1995 | 2013 | 0.69 (481/695) | 0.54 (309/567) | 1.45 | 2.6 | 0.01 | N | N | |
| ZS | M | 1993 | 2005 | 2013 | 0.69 (220/319) | 0.49 (113/231) | 1.86 | 2.6 | 0.007 | N | N | |
| Mitumba | EVA | F | 1965 | 2000 | 2010 | 0.62 (148/238) | 0.37 (88/235) | 1.72 | 2.0 | 0.04 | N | N |
aIn two of three age classes.
bIn later years.
Of the 35 males who reached adulthood before or during the study period (1976–2013) in Kasekela, there were six (AO, FG, FR, PX, SL, ZS) whose presence at a colobus encounter was positively associated with hunting probability, after controlling for adult male party size (table 3). At Mitumba, none of the six males was associated with increased hunting probability, after controlling for adult male and adult female party size. Given the significant association between female party size and hunting in this small population (see above), we also ran the same analyses for all adult females. Parties containing adult female EVA were more likely to hunt than those without her (estimate: 0.54, p = 0.04, table 3). We considered the six Kasekela males and the one Mitumba female as potential impact hunters before analysis of their individual hunting rates, below.
(b). Individual hunting frequency
(i). Kanyawara
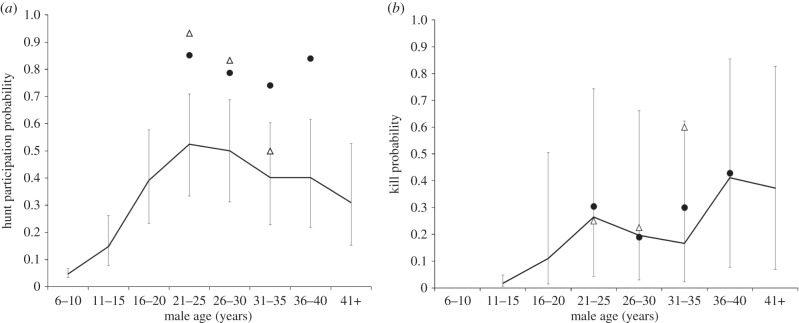
Individual hunting probability by age followed an inverted U-shaped distribution (figure 2a), although there was considerable variation within each age class. Older males in every age category were more likely to hunt than 6–10-year-olds were (GLMM, all p-values < 0.0001). 21–25-year-old males were most likely to hunt (52% of hunt attempts at which they were present), although this was not significantly higher than for any of the age categories between 16 and 40 years old (GLMM, all p ≥ 0.05). All males older than 15 were more likely to hunt than males in the younger categories (all p ≤ 0.05). Finally, males in the 40+ age group were significantly less likely to hunt than 21–15 and 26–30-year-olds (p ≤ 0.05). In short, at Kanyawara, males started to participate in hunts at maximum rates between the ages of 16 and 20, then showed a decline after age 40.
Figure 2.
Individual (a) hunting and (b) killing probability at Kanyawara. Lines represent predicted values from the GLMMs described in the text, with 1 s.e. error bars. Open triangles represent observed values for MS, solid circles for AJ.
At all ages for which there were data, AJ exhibited significantly higher (36–52% greater) hunting probability than the average male in the same age class (figure 2a, solid circles). MS (figure 2a, open triangles) exhibited higher hunting probability than the mean at ages 21–25 and 26–30, but showed average rates at age 31–35, suggesting a decline in hunting interest as a post-prime male. Therefore, we classified AJ as an impact hunter for all ages with data, and MS for ages 21–30 only. There are no data for either AJ or MS for age category 16–20 or younger, as colobus encounter data prior to 1996 are not available.
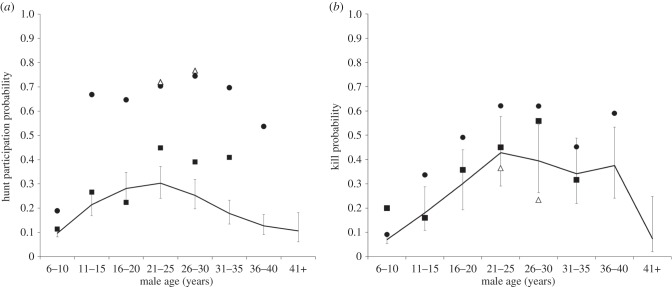
(ii). Kasekela
Similar to Kanyawara, 21–25-year-old Kasekela males had the highest hunting probability (figure 3a), but this value (0.3) was lower than at Kanyawara (0.52). Males in this age category were significantly more likely to hunt than males in all other age categories (all p < 0.003) except 16–20-year-olds (p = 0.20). The youngest (6–10) and oldest (36–40, 41+) males were least likely to hunt. Again, similar to Kanyawara, 16–20-year-old males were equally likely to hunt as males up to 30 years old. After 30, there was a significant decline in hunting probability. By age 36, males hunted at the same rates as 6–10-year-olds.
Figure 3.
Individual (a) hunting and (b) killing probability at Kasekela. Lines represent predicted values from the GLMMs described in the text, with 1 s.e. error bars. Open triangles represent observed values for FG, solid circles for FR, and solid squares for AO.
Of the six potential impact hunters identified earlier, ZS, PX and SL never exhibited hunting probabilities that were greater than the age-specific value predicted by the GLM described above. However, FG, FR and AO did exhibit higher than expected hunting probabilities. As a 21–25-year-old, FG's hunting probability was 138% greater than the mean (figure 3a, open triangles). As a 26- to 30-year-old, it was 203% greater than the mean. There are no data for FG as a younger male, and he died at age 29. FR (figure 3a, closed circles), who was followed for his whole life, exhibited significantly higher hunting probability at all ages, ranging from 96% to 322% higher than the mean. AO (figure 3a, closed squares) exhibited probabilities higher than the mean as a prime-aged (21–25, 26–30) and older (31–35) male, but not as a younger male. He died at age 34. We therefore classified FR as an impact hunter for his entire life, whereas AO was an impact hunter only in his prime. We do not know FG's early behaviour, but he was an impact hunter at the end of his life.
(iii). Mitumba
At Mitumba, there was little effect of age on male hunting probability. Six- to 10-year-old males were significantly less likely to hunt than 11- to 15-year-old males (GLMM, Z = 2.188, p = 0.03), which had the highest probability of hunting (0.15). However, there were no significant differences between hunting probabilities of 11- to 15-year-olds and males aged 21–25 (0.13) or 26–30 (0.11). Males aged 31–35 were significantly less likely to hunt (0.02) than other all age classes (all p < 0.02) except 6- to 10-year-olds (p = 0.30). There were only two hunts at which a male older than 36 was present; we excluded these data as the model did not converge. In sum, individual hunting probability was lower for males in Mitumba than Kasekela, but males reached peak hunting rates by 15 years old. There was very little variation in female hunting probability by age. With the exception of 26- to 30-year-olds, which had significantly lower hunting probability than all younger and older age classes (all p < 0.03), there were no significant differences among age classes (all p > 0.05). Overall, mean female hunting probability was 0.08 (range 0.03–0.11). Female EVA had an individual hunting probability of 0.13 at age 36–40, which fell within 1 s.e. of the expected value for similarly aged females. EVA was the only female in the sample who reached the 41+ age category.
(c). Prediction 1: impact hunters will initiate hunts more often than expected by chance
(i). Kanyawara
When he participated in a hunt, AJ was significantly more likely to be the first hunter than expected by chance, based on the number of other males that hunted (figure 4, exact Wilcoxon signed-ranks test, n = 8, V = 30, p (two-tailed) = 0.039). The same was also true for MS (figure 4, n = 8, V = 34, p (two-tailed) = 0.016). Furthermore, in the cases when one of them did NOT hunt first, it was highly likely that this was because the other one did. For example, there were 48 encounters when both were present and AJ did not hunt first. MS hunted first in 23 (48%) of these cases. Similarly, AJ hunted first in 24 (49%) of the 49 cases in which they were both present and MS did not hunt first. Indeed, when both AJ and MS were present, the probability that one of them was the first hunter was higher than expected (expected value = 2/X, where X = number of hunters, n = 7, V = 23, p (two-tailed) = 0.06, p (one-tailed) = 0.03)).
Figure 4.
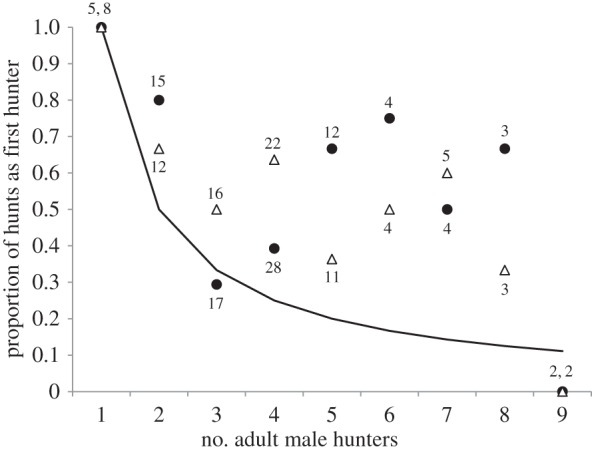
Probability of hunting first, Kanyawara. The line depicts the expected probability of hunting first, given the number of hunters. Solid circles indicate observed values for AJ, open triangles for MS. Numbers indicate sample sizes.
(ii). Kasekela
First hunter data have not yet been extracted from the narrative notes for the years when FG was alive. There were 46 hunts in which FR participated, multiple individuals hunted and the first hunter was clearly identified. FR was the first hunter in 40 (87%) of these cases. AO was the first hunter in 4 (31%) of the 13 hunts in which he was named as one of several hunters (and the first hunter was known).
(d). Prediction 2: when they hunt, impact hunters will be more likely to make a kill than expected for their age
(i). Kanyawara
No 6- to 10-year-old males at Kanyawara were observed to make a kill, and the GLMM would not converge when this age class was included in the model. After removing all 6- to 10-year-old males, we found that overall, individual hunting success increased with age, but there was considerable variation within each age class (figure 2b). Males in age classes older than 21–25 years were significantly more likely to make a kill than 11–15-year-olds (GLMM, all p < 0.01). Males in age classes 21–25, 36–40 and 41+ years were more likely to make a kill than 16–20-year-olds (all p < 0.02). Finally, the oldest males (36–40 and 41+ years) had higher kill rates than either 26–30 or 31–35-year-olds (all p < 0.02).
Neither AJ nor MS was more likely than expected to make a kill for any age class (figure 2b). When we re-ran the GLMM without including MS's data in calculations of the expected values, the observed probability that MS made a kill (0.6) at age 31–35 was greater than expected. This was not the case for AJ.
(ii). Kasekela
At Kasekela, the probability of making a kill followed an inverted-U-shaped function, peaking at age 21–25 (figure 3b). Males in this age category were more likely to make a kill than males in all other age classes (all p < 0.04) except 26–30 (p = 0.21) and 31–35 (p = 0.27). Six- to 10-year-old males were significantly less likely to make a kill than males in any other age class (GLMM, all p < 0.0003), except males older than 40 (p = 0.95). Similarly, kill probability by 11–15-year-olds was lower than that of all older age classes (all p < 0.00001) except males older than 40 (p = 0.35). 26–30-year-olds and 21–25-year-olds were more likely to make a kill than 16–20-year-olds (all p < 0.0009).
FR exhibited higher probability of success than expected at all ages except 31–35 (figure 3b, solid circles). By contrast, FG's success probability was no higher than expected (figure 3b, open triangles). AO's probability of success was higher than expected in two age categories (6–10, 26–30), but not in the other 4 (figure 3b, solid squares).
(e). Prediction 3: community-level hunting rates will decrease after an impact hunter dies or stops hunting at above average rates
(i). Kanyawara
AJ died at the very end of the study, therefore we are unable to assess the effect of his death on hunting rates. MS died in 2010. Hunting probabilities (hunt attempts/encounters) at each adult male party size were not significantly different in the 4 years following his death compared with the 4 previous years (exact Wilcoxon signed ranks test, n = 13, V = 34, p = 0.2). However, as MS's hunting rates were not unusually high between ages 31 and 35 (meaning that he was not an impact hunter during this period), we repeated this analysis, examining the 4-year period before and after MS's 31st birthday. In support of the prediction, we found that group hunting probability was significantly higher when MS was between the ages of 27 and 30 than when he was 31–34 (exact Wilcoxon signed ranks test, n = 9, V = 36, p = 0.007).
(ii). Kasekela
FG died in 1982. In support of prediction 3, hunting probabilities at each male party size were significantly lower in the 4 years after his death than in the previous 4 years (exact Wilcoxon signed-ranks test, V = 35, n = 8, p = 0.008). AO and FR both died in 2013, therefore we cannot yet determine the effect of their disappearance on hunting rates.
4. Discussion
This study highlights the importance of individual behavioural variation for catalysing cooperation. The results support the hypothesis that certain males initiate group hunts of colobus monkeys in two different chimpanzee communities. The ‘impact hunter’ hypothesis presents a more parsimonious alternative to the ‘collaboration’ hypothesis, which states that individuals engage in a ‘team task’ [44] in which some compromise their own chances of success for the sake of others’. We argue that group hunting by chimpanzees can be explained by a simple by-product mutualism in which each hunter is attempting to capture a monkey while his efforts incidentally improve the chances that other hunters will also succeed. Once a hunt begins, prey defense efforts are diluted, reducing hunting costs for other chimpanzees, and fleeing monkeys provide more opportunities for previously reluctant hunters to make a kill.
Over 18 years at Kanyawara, there were two impact hunters, adult males AJ and MS, whose presence at an encounter with colobus monkeys was associated (independently, cf. [21], which used a subset of the data in the present study) with an increased likelihood of hunting, regardless of party size. AJ participated in hunts more often than expected in all four age categories (ages 21–40) for which we had data. MS was more likely to hunt than the average male in his 20s, but not after age 31. Therefore, we did not classify MS as an impact hunter between age 31 and his death at 35. Over 37 years at Kasekela, there were six males whose presence was associated with increased hunting probability. We classified three of these males as impact hunters. FG and FR participated in hunts more frequently than similarly aged males over the whole period they were sampled (7 and 32 years, respectively). Because we had data on FG only in his prime (21–25 and 26–30 years old), it remains possible that his hunting rates had increased with age. AO's hunting proclivity developed in his prime—he hunted more than average between ages 21 and 35, but not as a younger male (ages 6–20). Thus, some males (FR, possibly AJ) were impact hunters for their whole adult lives, while others (AO, MS and possibly FG) varied in their hunting tendencies over time. Interestingly, FR was the only impact hunter who exhibited above average kill rates, which he did in every age category. In contrast, FG, AO, AJ and MS typically succeeded at or below the mean rate for males of their age. This suggests that while FR may have been particularly motivated to hunt because he was especially skilled, other factors must explain why the other males exhibited high hunting rates. For AO at least, the unusual hunting drive did not develop until he was in his 20s.
The impact hunter hypothesis hinges on the notion that these individuals hunt first, thus changing the pay-off structure for all other potential hunters. The data from Kanyawara strongly support this prediction. Both AJ and MS were more likely to initiate hunts than expected by chance (based on the number of other hunters). Furthermore, when one of them failed to hunt first, it was often because the other did. At Kasekela, in the cases in which the first hunter was recorded and FR hunted, he was the first hunter 87% of the time.
The impact hunter and collaboration hypotheses are not mutually exclusive. It is theoretically possible that the impact hunters at Kasekela and Kanyawara catalyse hunts by driving prey toward ‘ambushers’, as has been described at Taï. Indeed, this might explain why AJ, MS, AO and FG did not demonstrate unusually high success rates. However, Boesch [38] reported that collaboration was rare among the Kasekela chimpanzees. Collaboration also seems to be unusual at Kanyawara (R.W.Wrangham, personal observations, 1987–2014), Mahale [41] and Ngogo [40]. Boesch [38] attributes the high frequency of collaboration at Taï to the tall and uninterrupted forest canopy [36], which makes it intrinsically more difficult to capture prey. This explanation is consistent with Packer and Ruttan's [9] mathematical model, which predicts that cooperative hunting is most likely to evolve when solitary hunting success rates are low relative to hunting in groups. However, Gilby & Connor [45] argue that even the kind of division of labour observed at Taï can be explained by a by-product mutualism in which each hunter takes advantage of the actions of others. Unless it can be shown that individuals are not simply attempting to maximize their own chances of success by reacting to the movements of predators and prey, then the impact hunter/by-product mutualism explanation appears sufficient to explain cooperative hunting across chimpanzee populations.
Our support for the impact hunter hypothesis has important implications for our understanding of variation in cooperative behaviour within and between populations. Gilby et al. [21] proposed that the existence of impact hunters may explain temporal variation in hunting frequency within communities. In line with this prediction, we found that in Kasekela, hunting rates dropped significantly after FG's death. This was not the case at Kanyawara, however, as MS's death had no effect on hunting rates. However, MS was not an impact hunter when he was between the ages of 31 and 35. When we took this into account, we found that community-level hunting rates did indeed decrease after his impact hunter status was downgraded. FR, AO and AJ all died near the end of the study period; future work will indicate whether their departures affected hunting rates.
Additionally, this study provides preliminary data to test the prediction that the existence of an impact hunter can explain variation in hunting frequency between sites [21]. At Mitumba, where no single individual emerged as an impact hunter, hunting probability was lower (0.48) than at Kasekela (0.67), even though colobus encounter rates were remarkably similar. However, several other social and ecological factors likely contribute to this modest difference, including forest structure. A demographic explanation is that the Mitumba chimpanzees hunt less because there are fewer males in that community than at Kasekela. The majority of chimpanzee hunting studies, including this one, demonstrate that hunting probability is strongly positively correlated with male party size (reviewed in [14]).
Interestingly, our data indicate that females may be more active in hunts at Mitumba than at the other sites. Only at Mitumba did the number of females present at an encounter increase the likelihood of a hunt occurring. By re-running our models only on parties with fewer than five males at Kanyawara and Kasekela, we rejected the alternative explanation that this effect is only evident at Mitumba because of the paucity of males. Thus, increased participation by females may explain why hunting probability is still higher at Mitumba than at Kanyawara, even with less than half the number of males.
For the first time at Kanyawara, we found that after controlling for male party size, a hunt was less likely to occur if one or more sexually receptive females was present. An earlier study using a subset of the same data found a negative trend [21]. This result adds to the growing body of data that are inconsistent with the short-term meat-for-sex hypothesis [24], which proposes that chimpanzees hunt in order to provision sexually receptive females with meat in return for mating [68]. Instead, the negative association suggests that males forgo the chance for meat in favour of mate-guarding [24,39]. However, in this study, there was no statistically significant effect of swollen females on hunting probability at either Mitumba or Kasekela. This is in contrast to Gilby et al. [39], who found a similar negative relationship at Kasekela as we have now found at Kanyawara. Recent demographic changes (e.g. in the ratio of parous to nulliparous females or the number of cycling females) may explain this discrepancy. Nevertheless, over 37 years at Kasekela and 14 years at Mitumba, there was no evidence that the presence of swollen females increased hunting by males.
In sum, this study provides strong support from two chimpanzee communities that cooperative hunting can be explained by a simple by-product mutualism catalysed by the actions of certain impact hunters. Thus, individual variation in behaviour may explain how social species overcome the collective action problem they face before an ultimately mutually beneficial group-level behaviour is initiated. The biological and social underpinnings of such individual variation remain a fertile area for future research.
Acknowledgements
We are deeply grateful to the Gombe Stream Research Center staff for data collection, and to Dr Jane Goodall for granting us permission to work with the long-term dataset. We also thank Ross Bernstein, Jason Beyer, Kelly Hughes, Katie Lee, David Nunn and Anna Wynn for assistance with data extraction. We thank G. Isabirye-Basuta, J. Kasenene and J. Lwanga for their support of our work at Kanyawara. The late J. Barwogeza, the late J. Basigara, J. Sunday, C. Katongole, J. Kyomuhendo, F. Mugurusi, the late D. Muhangyi, the late C. Muruuli, S. Musana, J. Musunguzi, D. Sebugwawo, P. Tuhairwe, W. Tweheyo, R. Karamagi, D. Akaruhanga, S. Atwijuzee, E. Mugenyi and C. Abbe collected and extracted data, with research oversight by K. Duffy, C. Hooven, A. Houle, S. Mugume, E. Otali, K. Pieta and M. Wilson.
Many thanks to the two anonymous reviewers for helpful comments on an earlier version of the manuscript.
Ethics
This research complied with the laws of Tanzania and Uganda. Work at Gombe was approved by the Institutional Animal Care and Use Committee of Duke University, and by Tanzania National Parks, the Tanzania Wildlife Research Institute and the Tanzanian Commission for Science and Technology. Research at Kanyawara was approved by the Institutional Animal Care and Use Committees of Harvard University and the University of New Mexico, and by the Uganda Wildlife Authority, Uganda National Council of Science and Technology, and the Makerere University Biological Field Station.
Data accessibility
Data are available by contacting the corresponding author at ian.gilby@asu.edu.
Authors' contributions
I.C.G., Z.P.M. and R.W.W. designed the study; A.E.P., D.C.M., R.W.W. and M.N.M. oversaw data collection; I.C.G., Z.P.M., D.C.M. and J.R. compiled data; I.C.G. performed statistical analyses; all authors wrote the article.
Competing interests
We have no competing interests
Funding
Funding for long-term data collection at Gombe was provided by the Jane Goodall Institute, the National Science Foundation (DBS-9021946, SBR-9319909, BCS-0452315, IOS-LTREB-1052693, DGE-1106401), The National Institutes of Health (R01-AI058715, R00-HD057992), the University of Minnesota, the Harris Steel Group, the Windibrow Foundation, Minnesota Base Camp and Duke University. Research at Kanyawara was supported by the U.S. National Science Foundation (grants nos. 9807448, 0416125 and 1355014), the Leakey Foundation, the National Geographic Society, the Getty Foundation and the Wenner-Gren Foundation.
References
- 1.Olson M. 1965. The logic of collective action: public goods and the theory of groups. Cambridge, MA: Harvard University Press. [Google Scholar]
- 2.Clutton-Brock TH. 2009. Cooperation between non-kin in animal societies. Nature 462, 51–57. ( 10.1038/nature08366) [DOI] [PubMed] [Google Scholar]
- 3.Mathew S, Boyd R. 2014. The cost of cowardice: punitive sentiments towards free riders in Turkana raids. Evol. Hum. Behav. 35, 58–64. ( 10.1016/j.evolhumbehav.2013.10.001) [DOI] [Google Scholar]
- 4.Glowacki L, Wrangham R. 2014. Warfare and reproductive success in a tribal population. Proc. Natl Acad. Sci. USA 112, 348–353. ( 10.1073/pnas.1412287112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clements KC, Stephens DW. 1995. Testing non-kin cooperation: mutualism and the prisoner's dilemma. Anim. Behav. 50, 527–535. ( 10.1006/anbe.1995.0267) [DOI] [Google Scholar]
- 6.Mesterton-Gibbons M, Dugatkin LA. 1992. Cooperation among unrelated individuals: evolutionary factors. Q. Rev. Biol. 67, 267–281. ( 10.1086/417658) [DOI] [Google Scholar]
- 7.Gazda SK, Connor RC, Edgar RK, Cox F. 2005. A division of labour with role specialization in group-hunting bottlenose dolphins (Tursiops truncatus) off Cedar Key, Florida. Proc. R. Soc. B 272, 135–140. ( 10.1098/rspb.2004.2937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith TG, Siniff DB, Reichle R, Stone S. 1981. Coordinated behavior of killer whales, (Orcinus orca), hunting a crabeater seal (Lobodon carcinophagus). Can. J. Zool. 59, 1185–1189. ( 10.1139/z81-167) [DOI] [Google Scholar]
- 9.Packer C, Ruttan L. 1988. The evolution of cooperative hunting. Am. Nat. 132, 159–198. ( 10.1086/284844) [DOI] [Google Scholar]
- 10.Creel S, Creel NM. 1995. Communal hunting and pack size in African wild dogs, Lycaon pictus Anim. Behav. 50, 1325–1339. ( 10.1016/0003-3472(95)80048-4) [DOI] [Google Scholar]
- 11.Holekamp KE, Smale L, Cooper SM. 1997. Hunting rates and hunting success in the spotted hyena (Crocuta crocuta). J. Zool. 242, 1–15. ( 10.1111/j.1469-7998.1997.tb02925.x) [DOI] [Google Scholar]
- 12.Scheel D, Packer C. 1991. Group hunting behavior of lions: a search for cooperation. Anim. Behav. 41, 697–709. ( 10.1016/S0003-3472(05)80907-8) [DOI] [Google Scholar]
- 13.Muller MN, Mitani JC. 2005. Conflict and cooperation in wild chimpanzees. Adv. Stud. Behav. 35, 275–331. ( 10.1016/S0065-3454(05)35007-8) [DOI] [Google Scholar]
- 14.Newton-Fisher NE. 2014. The hunting behavior and carnivory of wild chimpanzees. In Handbook of paleoanthropology, vol. II (eds Henke W, Tattersall I), pp. 1–19. Berlin, Germany: Springer. [Google Scholar]
- 15.Bednarz JC. 1988. Cooperative hunting in Harris’ hawk. Science 239, 1525–1527. ( 10.1126/science.239.4847.1525) [DOI] [PubMed] [Google Scholar]
- 16.Marlowe F. 2010. The Hadza: hunter–gatherers of Tanzania. Berkeley, CA: University of California Press. [Google Scholar]
- 17.Packer C, Scheel D, Pusey AE. 1990. Why lions form groups: food is not enough. Am. Nat. 136, 1–19. ( 10.1086/285079) [DOI] [Google Scholar]
- 18.Creel S, Creel NM. 2002. The African wild dog: behavior, ecology, and conservation. Princeton, NJ: Princeton University Press. [Google Scholar]
- 19.Tennie C, O'Malley RC, Gilby IC. 2014. Why do chimpanzees hunt? Considering the benefits and costs of acquiring and consuming vertebrate versus invertebrate prey. J. Hum. Evol. 71, 38–45. ( 10.1016/j.jhevol.2014.02.015) [DOI] [PubMed] [Google Scholar]
- 20.Tennie C, Gilby IC, Mundry R. 2009. The meat-scrap hypothesis: small quantities of meat may promote cooperation in wild chimpanzees (Pan troglodytes). Behav. Ecol. Sociobiol. 63, 421–431. ( 10.1007/s00265-008-0676-3) [DOI] [Google Scholar]
- 21.Gilby IC, Eberly LE, Wrangham RW. 2008. Economic profitability of social predation among wild chimpanzees: individual variation promotes cooperation. Anim. Behav. 75, 351–360. ( 10.1016/j.anbehav.2007.06.008) [DOI] [Google Scholar]
- 22.Mitani JC. 2006. Reciprocal exchange in chimpanzees and other primates. In Cooperation in primates: mechanisms and evolution (eds Kappeler PM, van Schaik CP), pp. 101–113. Heidelberg, Germany: Springer. [Google Scholar]
- 23.Gilby IC. 2006. Meat sharing among the Gombe chimpanzees: harassment and reciprocal exchange. Anim. Behav. 71, 953–963. ( 10.1016/j.anbehav.2005.09.009) [DOI] [Google Scholar]
- 24.Gilby IC, Emery Thompson M, Ruane J, Wrangham RW. 2010. No evidence of short-term exchange of meat for sex among chimpanzees. J. Hum. Evol. 59, 44–53. ( 10.1016/j.jhevol.2010.02.006) [DOI] [PubMed] [Google Scholar]
- 25.Nunn CL. 2000. Collective benefits, free-riders, and male extra-group conflict. In Primate males (ed. Kappeler PM.), pp. 192–204. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 26.Marwell G, Ames RE. 1981. Economists free ride, does anyone else? J. Public Econ. 15, 295–310. ( 10.1016/0047-2727(81)90013-X) [DOI] [Google Scholar]
- 27.Isaac M, Walker J, Thomas S. 1984. Divergent evidence on free-riding: an experimental examination of possible explanations. Public Choice 43, 113–149. ( 10.1007/BF00140829) [DOI] [Google Scholar]
- 28.Sandler T. 1992. Collective action. Ann Arbor, MI: University of Michigan Press. [Google Scholar]
- 29.Wilson ML, et al. 2014. Lethal aggression in Pan is better explained by adaptive strategies than human impacts. Nature 513, 414–417. ( 10.1038/nature13727) [DOI] [PubMed] [Google Scholar]
- 30.Aureli F, et al. 2008. Fission–fusion dynamics: new research frameworks. Curr. Anthropol. 49, 627–654. ( 10.1086/586708) [DOI] [Google Scholar]
- 31.Nishida T. 1968. The social group of wild chimpanzees in the Mahali mountains. Primates 9, 167–224. ( 10.1007/BF01730971) [DOI] [Google Scholar]
- 32.Wrangham RW, Smuts B. 1980. Sex differences in the behavioral ecology of chimpanzees in the Gombe National Park, Tanzania. J. Reprod. Fertil. Suppl. 28, 13–31. [PubMed] [Google Scholar]
- 33.Goodall J. 1986. The chimpanzees of Gombe: patterns of behavior. Cambridge, MA: Harvard University Press. [Google Scholar]
- 34.Struhsaker TT. 2010. The red colobus monkeys: variation in demography, behavior and ecology of endangered species. Oxford, UK: Oxford University Press. [Google Scholar]
- 35.Watts DP, Mitani JC. 2002. Hunting behavior of chimpanzees at Ngogo, Kibale National Park, Uganda. Int. J. Primatol. 23, 1–28. ( 10.1023/A:1013270606320) [DOI] [Google Scholar]
- 36.Boesch C, Boesch-Achermann H. 2000. The Chimpanzees of the Taï Forest: behavioural ecology and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 37.Gilby IC. 2004. Hunting and meat sharing among the chimpanzees of Gombe National Park, Tanzania. PhD Thesis, University of Minnesota.
- 38.Boesch C. 1994. Cooperative hunting in wild chimpanzees. Anim. Behav. 48, 653–667. ( 10.1006/anbe.1994.1285) [DOI] [Google Scholar]
- 39.Gilby IC, Eberly LE, Pintea L, Pusey AE. 2006. Ecological and social influences on the hunting behaviour of wild chimpanzees (Pan troglodytes schweinfurthii). Anim. Behav. 72, 169–180. ( 10.1016/j.anbehav.2006.01.013) [DOI] [Google Scholar]
- 40.Mitani JC, Watts DP. 2001. Why do chimpanzees hunt and share meat? Anim. Behav. 61, 915–924. ( 10.1006/anbe.2000.1681) [DOI] [Google Scholar]
- 41.Hosaka K, Nishida T, Hamai M, Matsumoto-Oda A, Uehara S. 2001. Predation of mammals by the chimpanzees of the Mahale Mountains, Tanzania. In All apes great and small, vol. I. African apes (eds Galdikas B, Briggs N, Sheeran L, Shapiro G, Goodall J), pp. 107–130. New York, NY: Klewer Academic Publishers. [Google Scholar]
- 42.Boesch C. 2002. Cooperative hunting roles among Taï chimpanzees. Hum. Nat. 13, 27–46. ( 10.1007/s12110-002-1013-6) [DOI] [PubMed] [Google Scholar]
- 43.Boesch C, Boesch H. 1989. Hunting behavior of wild chimpanzees in the Taï National Park. Am. J. Phys. Anthropol. 78, 547–573. ( 10.1002/ajpa.1330780410) [DOI] [PubMed] [Google Scholar]
- 44.Anderson C, Franks NR. 2001. Teams in animal societies. Behav. Ecol. 12, 534–540. ( 10.1093/beheco/12.5.534) [DOI] [Google Scholar]
- 45.Gilby IC, Connor RC. 2010. The role of intelligence in group hunting: are chimpanzees different from other social predators? In The mind of the chimpanzee: ecological and experimental perspectives (eds Lonsdorf EV, Ross SR, Matsuzawa T), pp. 220–233. Chicago, IL: University of Chicago Press. [Google Scholar]
- 46.West-Eberhard MJ. 1975. The evolution of social behavior by kin selection. Q. Rev. Biol. 50, 1–33. ( 10.1086/408298) [DOI] [Google Scholar]
- 47.Brown JL. 1983. Cooperation: a biologist's dilemma. In Advances in behaviour (ed. Rosenblatt JS.), pp. 1–37. New York, NY: Academic Press. [Google Scholar]
- 48.Connor RC. 1995. The benefits of mutualism: a conceptual framework. Biol. Rev. Camb. Philos. Soc. 70, 427–457. ( 10.1111/j.1469-185X.1995.tb01196.x) [DOI] [Google Scholar]
- 49.Stander PE. 1992. Cooperative hunting in lions: the role of the individual. Behav. Ecol. Sociobiol. 29, 445–454. ( 10.1007/BF00170175) [DOI] [Google Scholar]
- 50.Kruuk H. 1972. The spotted hyaena: a study of predation and social behavior. Chicago, IL: University of Chicago Press. [Google Scholar]
- 51.Peterson RO, Ciucci P. 2003. The wolf as a carnivore. In Wolves: behavior, ecology and conservation (eds Mech LD, Boitani L), pp. 104–130. Chicago, IL: University of Chicago Press. [Google Scholar]
- 52.Bshary R, Hohner A, Ait-el-Djoudi K, Fricke H. 2006. Interspecific communicative and coordinated hunting between groupers and giant moray eels in the Red Sea. PLoS Biol. 4, 2393–2398. ( 10.1371/journal.pbio.0040431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gilby IC, Wilson ML, Pusey AE. 2013. Ecology rather than psychology explains co-occurrence of predation and border patrols in male chimpanzees. Anim. Behav. 86, 61–74. ( 10.1016/j.anbehav.2013.04.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gavrilets S, Fortunato L. 2014. A solution to the collective action problem in between-group conflict with within-group inequality. Nat. Commun. 5, 3526 ( 10.1038/ncomms4526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gavrilets S. 2015. Collective action problem in heterogeneous groups. Phil. Trans. R. Soc. B 370, 20150016 ( 10.1098/rstb.2015.0016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McAuliffe K, Wrangham R, Glowacki L, Russell AF. 2015. When cooperation begets cooperation: the role of key individuals in galvanizing support. Phil. Trans. R. Soc. B 370, 20150012 ( 10.1098/rstb.2015.0012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clutton-Brock TH, Gillett JB. 1979. A survey of forest composition in the Gombe National Park, Tanzania. Afr. J. Ecol. 17, 131–158. ( 10.1111/j.1365-2028.1979.tb00250.x) [DOI] [Google Scholar]
- 58.Altmann J. 1974. Observational study of behavior: sampling methods. Behaviour 49, 227–267. ( 10.1163/156853974X00534) [DOI] [PubMed] [Google Scholar]
- 59.Wilson ML. 2012. Long-term studies of the chimpanzees of Gombe National Park, Tanzania. In Long-term field studies of primates (eds Kappeler PM, Watts DP), pp. 357–384. Berlin, Germany: Springer. [Google Scholar]
- 60.Mjungu DC. 2010. Dynamics of intergroup competition in two neighboring chimpanzee communities. PhD thesis, University of Minnesota.
- 61.Strier KB, et al. 2010. The primate life history database: a unique shared ecological data resource. Methods Ecol. Evol. 1, 199–211. ( 10.1111/j.2041-210X.2010.00023.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chapman CA, Wrangham RW. 1993. Range use of the forest chimpanzees of Kibale: implications of the understanding of chimpanzee social organization. Am. J. Primatol. 31, 263–273. ( 10.1002/ajp.1350310403) [DOI] [PubMed] [Google Scholar]
- 63.Wilson ML, Kahlenberg SM, Wells M, Wrangham RW. 2012. Ecological and social factors affect the occurrence and outcomes of intergroup encounters in chimpanzees. Anim. Behav. 83, 277–291. ( 10.1016/j.anbehav.2011.11.004) [DOI] [Google Scholar]
- 64.R Development Core Team. 2015. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 65.Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, Dai B. 2014. lme4: linear mixed-effects models using Eigen and S4 See https://CRAN.R-project.org/package=lme4.
- 66.Hothorn T, Frank B, Westfall P. 2008. Simultaneous inference in general parametric models. Biometrical J. 50, 346–363. ( 10.1002/bimj.200810425) [DOI] [PubMed] [Google Scholar]
- 67.Gilby IC, Wrangham RW. 2007. Risk-prone hunting by chimpanzees (Pan troglodytes schweinfurthii) increases during periods of high diet quality. Behav. Ecol. Sociobiol. 61, 1771–1779. ( 10.1007/s00265-007-0410-6) [DOI] [Google Scholar]
- 68.Stanford CB, Wallis J, Mpongo E, Goodall J. 1994. Hunting decisions in wild chimpanzees. Behaviour 131, 1–18. ( 10.1163/156853994X00181) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available by contacting the corresponding author at ian.gilby@asu.edu.