Abstract
Introduction
The use of antiretroviral therapy (ART) has become a standard of care for the treatment of HIV infection. However, cost and resistance to ART are major obstacles for access to treatment especially in resource-limited settings. In this study, we aimed to assess the magnitude and associated factors of Immunological failure among adult and adolescent HIV infected Patients (with age ‘15yrs) on Highly Active Antiretroviral Therapy (HAART) in St. Luke and Tulu Bolo Hospitals, Oromia Region, Ethiopia.
Methods
A retrospective follow-up study was conducted among HIV-infected patients initiated 1st line ART at St. Luke and Tulu Bolo Hospitals, South West Shoa Zone, Oromia, Ethiopia.
Results
A total of 828 patient charts were reviewed. 477(57.6%) were female and the median age was 32 years. The median baseline CD4 count was 148cells/mm3. The most common prescribed ART was TDF based (36.7%). Out of 828 patients chart reviewed 6.8% (56) were developed immunological failure. Out of them only 20 (2.4%) were detected and put on second line regimen. The incidence of immunological failure was 1.8 cases per 100 person years of follow-up. Patients who had not disclosed their HIV status to any one had high risk of immunological failure compared with patients those who had disclosed their HIV status (AHR, 0.429; 95% CI 0.206 - 0.893; P-value=0.024).
Conclusion
Non disclosures of HIV status and with ambulatory of baseline functional status were found to be predictors of immunological failure. Most of the immunological failure cases were not detected early and not switched to second line ARV regimen. So patients with the above risk factors should be considered for a timely switch to second line HAART.
Keywords: HIV/AIDS, magnitude, risk factors, immunological, predictors, treatment failure
Introduction
The human immunodeficiency virus (HIV) epidemic continues to be a major challenge to global health. In 2011, an estimated 34 million people were living with human immunodeficiency virus /acquired immunodeficiency syndrome (HIV/AIDS) worldwide; of them 22.9 million were living in Sub-Saharan Africa [1]. According to 2011 Ethiopia Demographic Health survey (EDHS) and 2012 country report the estimated adult prevalence of HIV is 1.5% [2–4]. In Ethiopia considering the magnitude of the problem use of Highly Active Antiretroviral therapy (HAART) started in 2003 as a fee based treatment and then launched free ART in 2005 and by the end of January 2009 an estimated 180,447 clients had been enrolled in ART [5]. According to HEALTH SECTOR DEVELOPMENT PROGRAMME (HSDP) national report the total number of HIV positive people was estimated at 1,216,908 and out of them, 397,818 were eligible for ART, 333,434 ever started and 247,805 currently on ART [6]. According to 2010 Ethiopian national report only 865 adults and 13pediatric patients are on second line regimen which accounts only 0.6%. Immunological failure is defined as: fall of CD4 count to pre-therapy base line, 50% fall from the on treatment peak value, or persistent CD4 levels below 100cells/mm3 [4]. Knowing factors that can help to predict treatment failure will help to identify clients that are at higher risk of treatment failure so as to change regimen for those who already have failed regimen and delay though maximizing follow in those have potential failure. Routine patient follow-up need to be done as part of the routine clinical follow up and need to have a high index of suspension. Studies shows that only a few patients among those who actually had treatment failure were detected at the ART centers. The mean duration of treatment failure on first line ART and the mean duration from detection of treatment failure to switch to second line ART were longer [3]. Different studies from South Africa, Malawi, Ethiopia and Haiti revealed that the prevalence of treatment failure range from 9.8% -15% [6, 3]. There are various risk factors described in different studies and world health Organization (WHO) guidelines [7]. Yet published data on determining of magnitude and associated factors of Immunological failure are limited. Risk factors for Immunological failure have not been studied in this area. So the aim of this study was to assess the magnitude and associated factors of Immunological failure among adult and adolescent HIV infected Patients (with age ≥15yrs) on HAART and to provide information to optimize assessing of Immunological failure among HIV patients on HAART.
Methods
Study area and period
The study was conducted in St. Luke and Tulubolo Hospitals, south west Shoa zone, Oromia region St. Luke hospital is located in Woliso town and at distance of 125 Km and Tulubolo hospital is located in Tulubolo town which is 95 km away from Addis Ababa, the capital city of Ethiopia. St. Luke Hospital ever enrolled HIV positive clients/patients to chronic HIV care starting from April 2006-July 2013 who's age ≥15 years is 3478 (F=2134, M=1344) and patients ever started on Highly Active Antiretroviral Therapy (HAART) for the same age and period is 2126 (F=1225, M=901). For Tulubolo Hospital number of patients ever enrolled in HIV chronic care starting from May 2010-July 2013 (the Hospital has started ART service in May 2010) who's age ≥15 years is 218 (F=142, M=76) and patients put on Highly Active Antiretroviral Therapy (HAART) for the same age is 130 (F=82, M=48) and the study period was from April 2006 up to July 2013.
Study design
Institutional based retrospective follow up study was carried out among HIV-positive patients on HAART for ≥12 months. Hence the chart of HIV positive patients on HAART for ≥12 months between April 2006 and July 2013 were reviewed retrospectively.
Source population
Source population was all human immune Deficiency Virus (HIV) infected patients with age ≥15yrs who were on first line Antiretroviral Therapy (ART) regimen in St. Luke and Tulu Bolo Hospitals.
Study population
The study population was all HIV infected patients with age ≥15yrs who started first line ART in St. Luke and Tulu Bolo Hospitals since April 2006-July 2013.
Sample size determination Double proportional formula
nj=Zα/2√(1+1/r)P(1-P)+Zβ√P1(1-P1)+P2(1-P2)/r (P1-P2)2
was used to determine the sample size of the study; EPI-info version 3.5.3 window is used with type 1 error 5%, power of 80% and ratio of exposed to unexposed 1:1, and the sample size has been calculated for exposure status in different variables using the following formula. Proportion of exposure status in these variables is taken from previous studies [8, 9]. And the final sample size for the study was 828.
Sampling procedure
Two hospitals which are found in south west shoa zone were used. From both hospitals HIV patients on HAART for ≥12 months has been were selected. The calculated final sample size was 828 with zero none response rate. Systematic random sampling is employed after proportional allocation of the sample for both Hospitals.
Study variables
The dependent variable of the study was immunologic failure. The independent variables include Socio demographic characteristics, Behavioral status and Base line clinical and Laboratory information.
Data collection methods and instrument used
To ensure consistency of the data electronic data base, registrations and patient charts was used to extract the data. The data were collected by preparing chart abstraction form.
Data quality control
Before data collection the data collectors and supervisors were get a one day orientation by principal investigator. The data collectors were Hospitals data clerks and the supervisors were Hospitals ART providers. There was a regular supervision to data collectors by the principal investigator and supervisors to maintain the data quality.
Data analysis
The data was entered to Epi-data 3.1 version and analyzed using SPSS version 20. Descriptive analysis using frequency and summary statistics was conducted to describe the study subject. Immunologic failure distribution within groups in respect to time after initiation of therapy is estimated using Kaplan Meier method and log rank tests. Bivariate Cox regression analysis has been used to determine the association between independent variables and covariates with significant association (p value
Ethical consideration
The study was approved by an ethical committee in Addis Continental Institute of Public Health. A support letter to the two Hospitals was obtained from the institute before conducting the data collection. The information that was collected by the study were stored in a file and kept confidential.
Results
Base line socio demographic and socio clinical character: from a total of 828 patients data reviewed retrospectively 57.6% (477) of them were female. 61.1% (506) were married and followed by 14.7% (122) widower, 10.4% (86) never married. The median age of the participant at enrollment was 32.00 year (IQR, 27-39yrs) and 45.7% (378) go through primary education (Table 1).
Table 1.
Baseline socio demographic characteristics of HIV patients on HAART in St.Luke and Tulubolo Hospitals, April 2006-July 2013.(n=828),Ethiopia
| Variable | Frequency | Percent |
|---|---|---|
| Age | ||
| 15-24 | 81 | 9.8 |
| 25-34 | 377 | 45.5 |
| 35-44 | 253 | 30.6 |
| >=45 | 117 | 14.1 |
| Sex | ||
| Male | 351 | 42.4 |
| Female | 477 | 57.6 |
| Marital status | ||
| Never Married | 86 | 10.4 |
| Married | 506 | 61.1 |
| Divorced | 44 | 5.3 |
| Separated | 70 | 8.5 |
| Widowed | 122 | 14.7 |
| Educational status | ||
| No Education | 198 | 23.9 |
| Primary | 378 | 45.7 |
| Secondary | 214 | 25.8 |
| Tertiary | 38 | 4.6 |
| Patient referral | ||
| From within the facility or hospital | 688 | 83.1 |
| From outside the facility | 140 | 16.9 |
| Employment status | ||
| Employed | 279 | 33.7 |
| Unemployed | 549 | 66.3 |
| Religion | ||
| Orthodox | 530 | 64.0 |
| Muslim | 105 | 12.7 |
| Protestant | 175 | 21.1 |
| Catholic | 12 | 1.4 |
| ^Others | 6 | 0.7 |
| Condition of spouse | ||
| Healthy | 175 | 21.1 |
| Chronically ill Dead |
221 102 |
26.7 12.3 |
| Unknown | 255 | 30.8 |
Patients other than mentioned religions
Base line clinical and laboratory information: about 82.5% of patients were working in their functional status at a time of enrollment. The commonest documented OI at time of enrollment were herpes Zoster, followed by oral thrush and TB which accounts 22.3%, 15.8% and 13.7% respectively. At base line the median CD4 count was 148cells/mm3 (IQR, 100-200) and the mean Hemoglobin was 12.08g/dl (SD, 1.934). 99.5%(824) were given cotrimoxzole at the time of ART initiation. At base line 33.5%(278) of the participants were on WHO staging 3 and 4. Thirty nine point two percent (325) of participants know their spouse HIV status (Table 2).
Table 2.
Base line clinical and laboratory information of HIV patients on HAART in St.Luke and Tulu Bolo Hospitals, Ethiopia, April 2006-July 2013 (N=828)
| Variable | frequency | Percent |
|---|---|---|
| Weight | ||
| < 50 | 461 | 55.7 |
| ≥ 50 | 367 | 44.3 |
| Functional status | ||
| Working | 670 | 80.9 |
| Ambulatory | 133 | 16.1 |
| Bedridden | 25 | 3.0 |
| WHO stage | ||
| Stage 1 | 281 | 33.9 |
| Stage 2 | 316 | 38.2 |
| Stage 3 | 183 | 22.1 |
| Stage 4 | 48 | 5.8 |
| Base line CD4 | ||
| ≤ 200cell/mm3 | 619 | 74.8 |
| > 200cell/mm3 | 201 | 24.3 |
| Base line CPT | ||
| Yes | 824 | 99.5 |
| No | 4 | 0.5 |
| Base line TB | ||
| Yes | 109 | 13.2 |
| No | 719 | 86.8 |
| Base line Hgb | ||
| < 9 g/dl | 53 | 6.4 |
| 9-11 g/dl | 207 | 25 |
| > 11 g/dl | 568 | 68.6 |
Magnitude of Immunological failure: a total of 828 patients were followed retrospectively for a median time of 44 months (IQR 32-56) with a minimum of 18 and maximum 97 months follow up from April 2006-July 2013. Out of 828 patients 6.8% (56) developed immunological failure. The incidence of immunological failure was 1.8 cases per 100 person years of follow-up.
Time of Immunological failure: the median time of failure for those qualified immunological failure was 48 months (IQR 35-65 months). Life table showed treatment failure started by 24 month follow-up with 16% and similarly 13%, 6%, 26%, 14%, 12% for the month of 30, 36, 42, 48, and 54 respectively. A sharp drop is seen at 84th month. Life table distribution and the survival function curve is shown in Table 3 and Figure 1. The findings of survival functions between groups were compared using Kaplan-Meier method. To see the significant differences between the groups the Log rank test was used and the result is shown in Table 4, Table 5.
Table 3.
Life table distribution of probability of immunological failure of patients on HAART St.Luke and Tulu Bolo Hospital, Ethiopia, April 2006-July 2013
| *Interval Start Time | Number Entering Interval | Number Withdrawing during Interval | Number Exposed to Risk | Number of Terminal Events | Proportion Terminating | Proportion Surviving | Cumulative Proportion Surviving at End of Interval |
|---|---|---|---|---|---|---|---|
| 0 | 65 | 0 | 65.000 | 0 | 0.00 | 1.00 | 1.00 |
| 6 | 65 | 0 | 65.000 | 0 | 0.00 | 1.00 | 1.00 |
| 12 | 65 | 0 | 65.000 | 0 | 0.00 | 1.00 | 1.00 |
| 18 | 65 | 0 | 65.000 | 0 | 0.00 | 1.00 | 1.00 |
| 24 | 65 | 1 | 64.500 | 10 | 0.16 | 0.84 | 0.84 |
| 30 | 54 | 0 | 54.000 | 7 | 0.13 | 0.87 | 0.74 |
| 36 | 47 | 1 | 46.500 | 3 | 0.06 | 0.94 | 0.69 |
| 42 | 43 | 2 | 42.000 | 11 | 0.26 | 0.74 | 0.51 |
| 48 | 30 | 1 | 29.500 | 4 | 0.14 | 0.86 | 0.44 |
| 54 | 25 | 0 | 25.000 | 3 | 0.12 | 0.88 | 0.39 |
| 60 | 22 | 1 | 21.500 | 8 | 0.37 | 0.63 | 0.24 |
| 66 | 13 | 2 | 12.000 | 1 | 0.08 | 0.92 | 0.22 |
| 72 | 10 | 0 | 10.000 | 5 | 0.50 | 0.50 | 0.11 |
| 78 | 5 | 0 | 5.000 | 3 | 0.60 | 0.40 | 0.04 |
| 84 | 2 | 1 | 1.500 | 1 | 0.67 | 0.33 | 0.01 |
Six month interval was taken from the WHO and National guide line which is recommended for immunological follow-up
Figure 1.
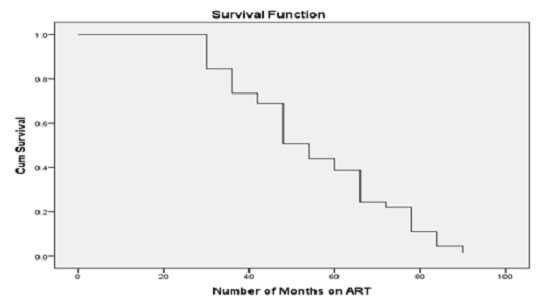
Cumulative probability of not developing immunological failure for patients after initiation of HAART, St.Luke and Tulu Bolo Hospital, April 2006-July 2013
Table 4.
Kaplan-Meier and log rank analysis for comparison of survival time for patients on HAART according to base line information, April, 2006-July, 2013. (N=828)
| Variable | Mean survival | 95% Confidence Interval | ||
|---|---|---|---|---|
| Lower | Upper | Log rank test | ||
| Sex | 0.083 | |||
| Male | 54.8 | 48.1 | 61.6 | |
| Female | 47.6 | 41.3 | 53.9 | |
| Age | 0.56 | |||
| 15-24 | 44.4 | 33.9 | 54.8 | |
| 25-34 | 52.9 | 44.6 | 61.2 | |
| 35-44 | 51.8 | 44.3 | 59.3 | |
| >=45 | 51.7 | 40.5 | 62.9 | |
| Education | 0.056 | |||
| NoEducation | 47.5 | 37.8 | 57.1 | |
| Primary | 48.0 | 41.4 | 54.7 | |
| Secondary | 56.8 | 47.9 | 65.6 | |
| Teritiary | 77.6 | 67.5 | 87.8 | |
| Marital status | 0.67 | |||
| Never Married | 47.3 | 34.1 | 60.5 | |
| Married | 52.4 | 46.3 | 58.4 | |
| Divorced | 61.5 | 59.4 | 63.5 | |
| Separated | 51.0 | 44.4 | 57.5 | |
| Widowed | 47.3 | 32.2 | 62.3 | |
| Patient referral | 0.48 | |||
| From within the facility or hospital | 50.6 | 45.5 | 55.7 | |
| From outside the facility | 63.0 | 53.1 | 72.8 | |
Table 5.
Kaplan-Meier and Log rank analysis for comparison of survival time for patients on HAART according to base line information, April, 2006-July, 2013. (N=828)
| Variable | Mean survival | 95% Confidence Interval | Log rank test | |
|---|---|---|---|---|
| Lower | Upper | |||
| Disclosure status | 0.016# | |||
| Yes | 54.4 | 48.3 | 60.5 | |
| No | 45.3 | 39.5 | 51.1 | |
| Weight at baseline | 0.23 | |||
| < 50 | 53.3 | 46.9 | 59.6 | |
| >=50 | 49.0 | 42.1 | 55.9 | |
| WHO stage at base line | 0.3 | |||
| Stage1 | 45.4 | 36.0 | 54.9 | |
| Stage2 | 54.2 | 48.1 | 60.4 | |
| Stage3 | 52.9 | 40.0 | 65.7 | |
| Stage4 | 64.5 | 54.7 | 74.2 | |
| OI at base line | 0.17 | |||
| Yes | 53.9 | 48.8 | 59.0 | |
| No | 45.6 | 34.6 | 56.7 | |
| Base line CD4 | 0.013# | |||
| <=200 | 54.9 | 48.9 | 60.9 | |
| > 200 | 43.5 | 37.0 | 50.0 | |
| Functional status | 0.2 | |||
| Working | 49.3 | 44.2 | 54.3 | |
| Ambulatory | 61.7 | 50.9 | 72.4 | |
| Bedridden | 50.5 | 8.3 | 92.6 | |
| Past TB treatment | 0.15 | |||
| Yes | 63.375 | 50.7 | 76.0 | |
| No | 49.964 | 45.0 | 54.8 | |
p-value <0.05
Predictors of Immunological failure: in bivariate Cox regression CD4 count and disclosure of HIV status were statistically significant. Significance difference was seen for two variables. When survival time for disclosure status was compared, those patients who didn't disclose their HIV status to family member had significantly lower mean survival time, 45.3 with P value=0.016. Significant difference was seen between CD4 count below or equal to 200cell/mm3 and greater than 200cell/mm3, time to failure in the latter group was shorter (P value=0.013). No significant association was observed between age, sex, educational status, employment status, marital status, and base line weight of the patient, presence of OI, baseline WHO stage, baseline functional status, presence of past TB and time to Immunological failure. The variable that were found to be significantly associated with immunological failure in bivariate and those with P-value ≤0.2 in Bivariate Cox regression were entered in to multivariate Cox-model. Age and sex was also entered in to the model irrespective of their association to immunological failure as they are the commonest confounders. The multivariate model analysis made evident that disclosure status was significantly associated with immunological failure at P-value less than 0.05 and from the Bivariate Cox regression, functional status was found to be significantly associated with immunological failure. Patients who didn't disclose their HIV status to any one had high risk of developing immunological failure compared with patients those who had disclose their HIV status (AHR, 0.429; 95% CI 0.206 - 0.893; P-value=0.024). Patients with base line functional status ambulatory had risk of developing immunological failure than patients with working and bedridden functional status (HR, 0.337; 95% CI 0.137-0.828; P-value=0.018) (Table 6, Table 7).
Table 6.
Cox proportional hazard analysis with bivariate and multivariate model for socio demographic, clinical and laboratory, HIV patients on HAART in St.Luke and Tulu Bolo Hospital, Ethiopia, from April 2006-July 2013. (n=828)
| Variable | Immunological failure (%) | Immunological success (%) | Crude HR (95% CI) | Adjusted HR#(95% CI) |
|---|---|---|---|---|
| Sex | 0.737(0.323-1.683) | |||
| Male | 30(8.5) | 321(91.5) | 1 | |
| Female | 26(5.5) | 451(94.5) | 0. 627 (0.365-1.075 | |
| Age | ||||
| 15-24 | 5(6.2) | 76(93.8) | 1 | |
| 25-34 | 23(6.1) | 354(93.9) | 1.847 (0.610-5.591) | |
| 35-44 | 19(7.5) | 234(92.5) | 0.913 (0.6416-2.00) | |
| >=45 | 9(7.7) | 108(92.3) | 0.978 (0.440-2.172) | |
| Education | ||||
| No Education | 12(6.1) | 186(93.9) | 1 | |
| Primary | 30(7.9) | 348(92.1) | 1.023 (0.543-2.040) | |
| Secondary | 13(6.1) | 201(93.9) | 0.635 (0.284-1.418) | |
| Teritiary | 1(2.6) | 37(97.4) | 0.125 (0.016-0.967) | |
| Patient referral | ||||
| From within the hospital | 50(7.3) | 638(92.7) | 1 | |
| From outside the facility | 6(4.3) | 134(95.7) | 1.352 (0. 574 -3.180) |
HR- Hazard Ratio, @ Statistically significant
Table 7.
Cox proportional hazard analysis with bivariate and multivariate model for socio demographic, clinical and laboratory, HIV patients on HAART in St.Luke and Tulu Bolo Hospital, Ethiopia from April 2006-July 2013. (n=828)
| Variable | Immunological failure (%) | Immunological success (%) | Crude HR (95% CI) | Adjusted HR# (95% CI) |
|---|---|---|---|---|
| Disclosure status | ||||
| Yes | 38(6.8) | 517(93.2) | 2.056(1.120-3.774) | 0.429(0.206-0.893)@ |
| No | 18(6.6) | 255(93.4) | 1 | 1 |
| WHO stage | ||||
| Stage 1 | 17(6.1) | 264(93.9) | 1 | |
| Stage 2 | 25(7.9) | 291(92.1) | 1.916 (0.637-5.761) | |
| Stage3 | 10(5.5) | 173(94.5) | 1.182(0.408-3.426) | |
| Stage4 | 4(8.3) | 44(91.7) | 1.124 (0. 342-3.688) | |
| Base line CD4 count (N=820) | 0.517(0.253-1.057) | |||
| <=200 | 38(6.1) | 581(93.9) | 0.469 (0.252-0.872) | |
| > 200 | 16(8) | 185(92) | 1 | |
| Functional status | ||||
| Working | 43(6.4) | 627(93.6) | 1 | 1 |
| Ambulatory | 11(8.3) | 102(91.7) | 0. 557 (0.274-1.130) | 0.337(0.137-0.828)@ |
| Bedridden | 2(8) | 23(92) | 1.044 (0 .250-4.354) | 0.599(0.111-3.216) |
| Weight | ||||
| <50 | 34(7.4) | 427(92.6) | 0.718 (0.412-1.253) | |
| >=50 | 22(6) | 345(94) | 1 |
HR- Hazard Ratio
Statistically significant
Discussion
In this retrospective follow-up study Patients on follow-up contributed a total of 3116 person years and incidence of immunological failure was 1.8 per 100 person years of follow-up (PYFUS). From the study found that the independent significant predictors of Immunological failure in patients living with HIV/AIDS after initiation of ART were not disclosing of HIV status to any one of family member and being ambulatory in baseline functional status. As per the WHO and/national ART guide line definition 6.8% of patients had developed immunological failure. The finding is consistent with the finding from India where the prevalence of Immunological failure is 7.3% in participants who had been on HAART for a median of 17 months (IQR:6-30 months) and study conducted in Kenya which shows immunological failure 5.7% [10–13]. The median pre HAART CD4 count observed in this study is 148cell/ mm3 which is also comparable with studies conducted in Djibuti with median CD4 count 144cell/mm3 and study conducted in Tanzania with median CD4 count 159 cells/mm3 (IQR; 65-234) [8, 11, 14]. In bivariate cox regression base line CD4 count ≥ 200/mm3 was statistical association in Immunological failure, In contrast studies conducted in India and other sites showed that low Cd4 count (13,8].
Limitation of the study
This study was conducted retrospectively on Patients chart review. So that most reviewed charts have got missed laboratory tests like platelet counts and pre HAART viral load determination was not included in the study due to absence of Viral load laboratory test in the hospitals.
Conclusion
According to this study the majority of patients with immunological failure were not diagnosed timely and not switched to second line regimen. So knowing the predictor factors and timing of failure are important parts for care and treatment. Having ambulatory functional status at base line and disclosing of HIV status to family members has identified the independent significant predictors of immunological failure in patients living with HIV/AIDS after initiation of HAART by this study. Based on the finding, the following recommendations were forwarded. Baseline CD4 counts >200cell/mm3 should needs further study, sensitization of health care providers to focus on immunological failure and health care providers should be guided to focus on predictors of immunological failure.
Acknowledgments
First of all we would like to thank to our almighty GOD who giving us good health throughout our life. We grateful to Haromaya University, Department of Public Health and Addis Continental Institute of Public Health (ACIPH). Our thanks also extends to those all who cooperated with us in doing this work.
Competing interests
The authors declare no competing interest.
Authors’ contributions
Bekelech Bayou conceived and designed the study and collected data, performed analysis, interpretation of data. Dr. Abera Kumie and Abay Sisay had assisted with the design, performed, analysis, interpretation of data, drafted and critically reviewed of this manuscript.
References
- 1.Tadewos A, Addis Z, Ambachew H, Banerjee S. Prevalence of dyslipidemia among HIV-infected patients using first-line highly active antiretroviral therapy in Southern Ethiopia: a cross-sectional comparative group study. AIDS Research and Therapy. 2012;9(31) doi: 10.1186/1742-6405-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Federal Democratic Republic of Ethiopia. Federal HIV/AIDS Prevention and control Office. Addis Ababa, Ethiopia: Management in Ethiopia; 2009. Guidelines for Implementation of HIV/AIDS Case; p. 1. [Google Scholar]
- 3.Bacha T, Tilahun B, Worku A. Predictors of treatment failure and time to detection and switching in HIV-infected Ethiopian children receiving first line anti-retroviral therapy. BMC Infectious Diseases. 2012;12(197) doi: 10.1186/1471-2334-12-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Federal Democratic Republic of Ethiopia. Addis Ababa, Ethiopia: Ministry of Health; 2012. Country progress report on HIV/AIDS response. [Google Scholar]
- 5.Federal Democratic Republic of Ethiopian. Ministry Of Health. Ethiopia: Addis Ababa; 2010/2011. Health sector development programme IV version 1 annual performance report; p. 28 & 30. [Google Scholar]
- 6.Charles M, Leger P, Severe P, Guiteau C, Apollon A, Gulick R, et al. Virologic, clinical and immunologic responses following failure of first-line antiretroviral therapy in Haiti. Journal of the International AIDS Society. 2012;15(2):173–75. doi: 10.7448/IAS.15.2.17375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonathan Z. Minority HIV-1 Drug Resistance Mutations and the Risk of NNRTI-based Antiretroviral Treatment Failure: A Systematic Review and Pooled Analysis. Journal of American medical association. 2011;305(13):1327–1335. doi: 10.1001/jama.2011.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khienprasit N, Chaiwarith R, Sirisanthana T, Supparatpinyo K. Incidence and risk factors of antiretroviral treatment failure in treatment-naïve HIV-infected patients at Chiang Mai University Hospital, Thailand. AIDS Research and Therapy. 2011;8(1):42. doi: 10.1186/1742-6405-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kassa D, Gebremichael G, Alemayehu Y, Wolday D, Messele T, Baarle D. Virologic and immunologic outcome of HAART in Human Immunodeficiency Virus (HIV)-1 infected patients with and without tuberculosis (TB) and latent TB infection (LTBI) in Addis Ababa, Ethiopia. AIDS Research and Therapy. 2013;1742(6405):10–18. doi: 10.1186/1742-6405-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liaoa L, Xinga H, Sub B, Wangc Z, Ruana Y, Wanga X, et al. Impact of HIV drug resistance on virologic and immunologic failure and mortality in a cohort of patients on antiretroviral therapy in China. AIDS. 2013;27(11):1815–24. doi: 10.1097/QAD.0b013e3283611931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma Y, Zhao D, Yu L, Bulterys M, Robinson M, Zhao Y, et al. Predictors of virologic failure in HIV-1-infected adults on first line antiretroviral therapy in eight provinces in China. Clinical Infectious Diseases. 2010;50(2):264–71. doi: 10.1086/649215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh A, Agarwal A, Chakravarty J, kumari S, Rai M, et al. Predictive Markers of Failure of First Line Anti Retroviral Treatment in HIV Patients in India. J AIDS Clin Res. 2013;4(210) [Google Scholar]
- 13.Ferreyra C, Yun O, Eisenberg N, Alonso E, Khamadi A, Mwau M, et al. Evaluation of Clinical and Immunological Markers for Predicting Virological Failure in a HIV/AIDS Treatment Cohort in Busia, Kenya. PLoS ONE. 2012;7(11):e49834. doi: 10.1371/journal.pone.0049834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ElmiAbar A, Jlizi A, Youssouf H, Ali M, Hadj B, Slim A. HIV-1 drug resistance genotyping from antiretroviral therapy (ART) naïve and first-line treatment failures in Djiboutian patients. Diagnostic Pathology. 2012;7(138) doi: 10.1186/1746-1596-7-138. [DOI] [PMC free article] [PubMed] [Google Scholar]


